-
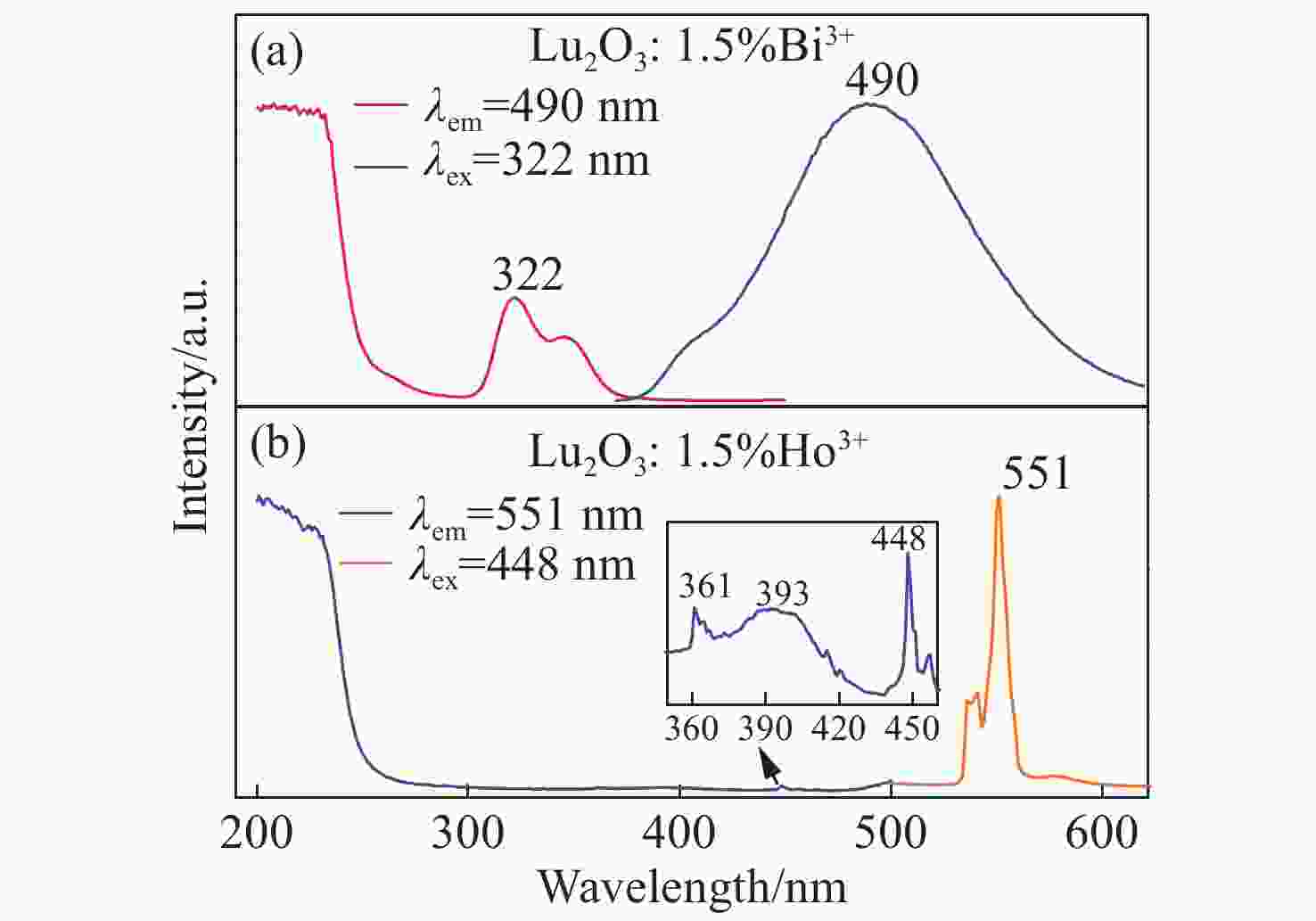
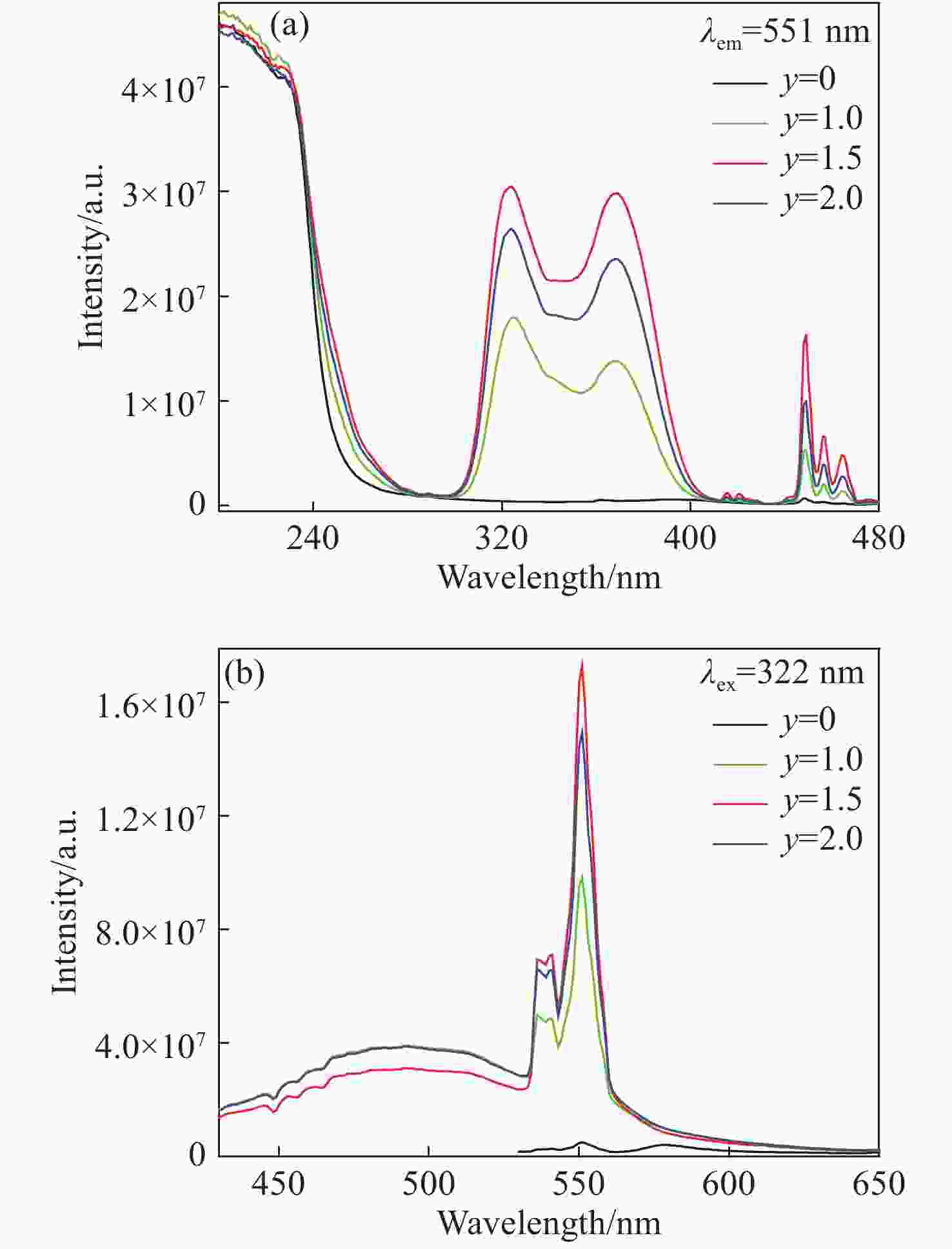
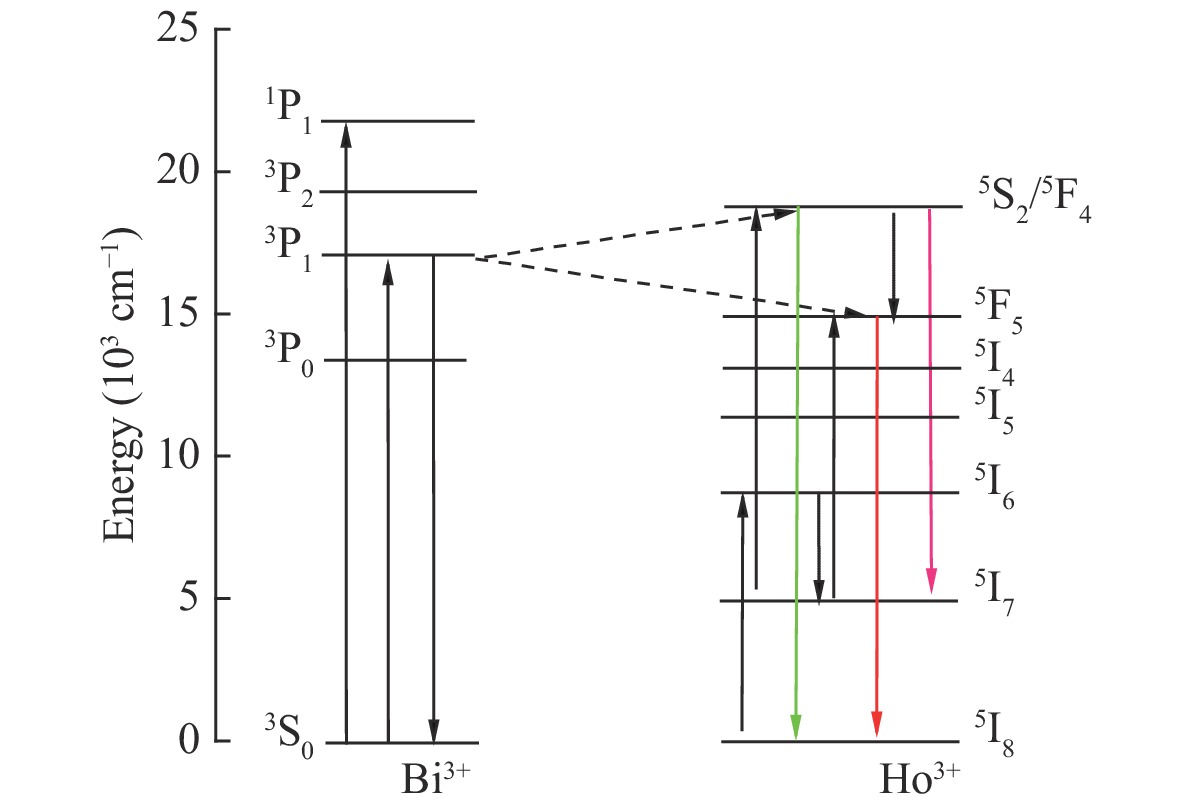
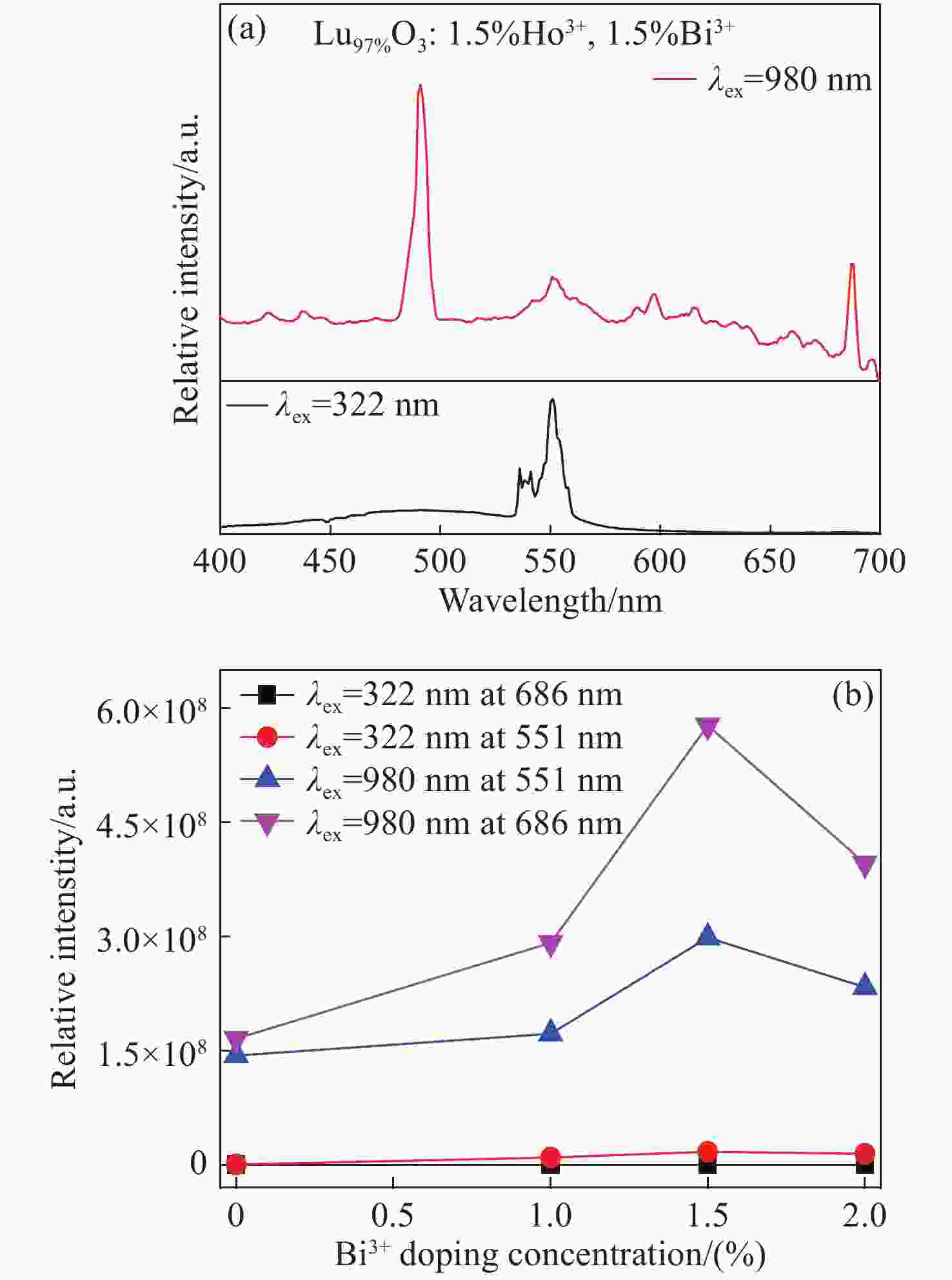
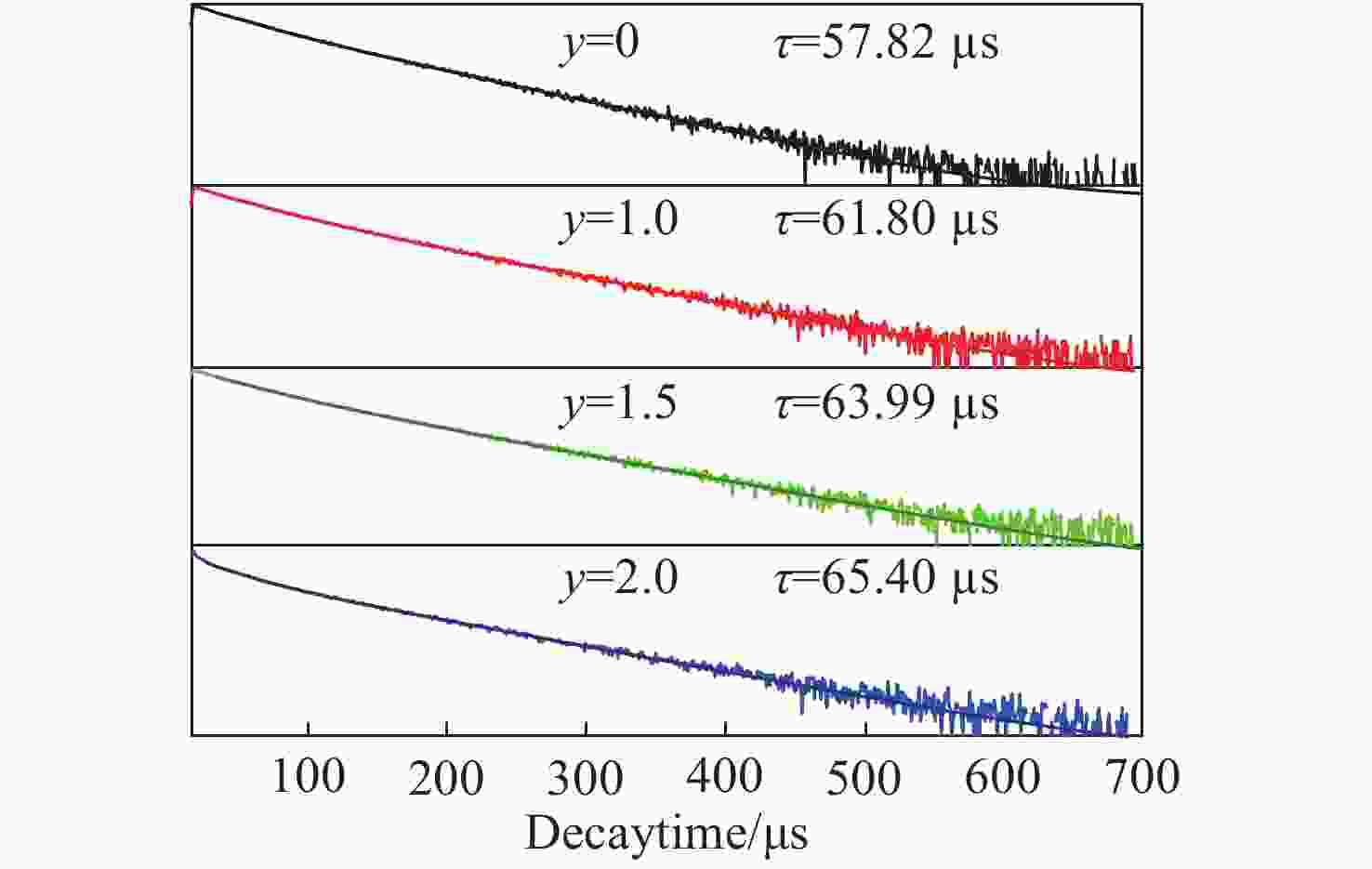
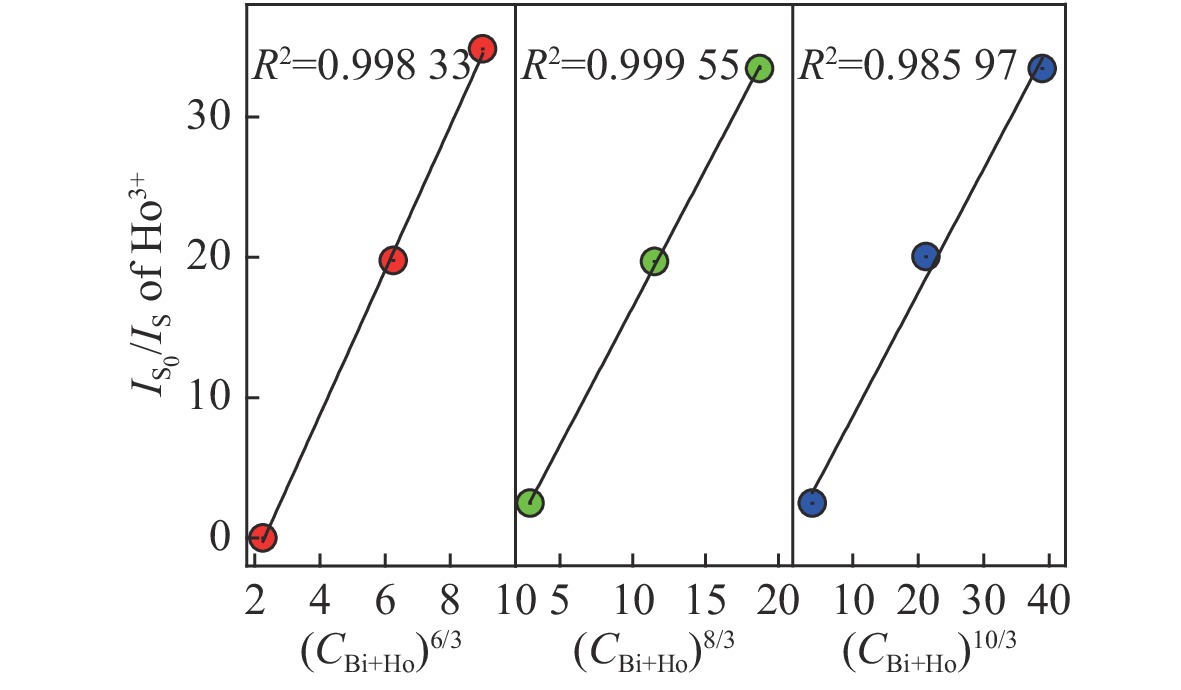
摘要: 采用高温固相法制备了金属离子Bi3+掺杂Lu1-xO3: x%Ho3+系列荧光粉,研究了不同浓度Bi3+掺杂Lu1-xO3: x%Ho3+荧光粉的晶体结构、Lu2O3基质中Bi3+→Ho3+的能量传递规律及合成粉体的发光性质。X射线衍射结果表明Bi3+、Ho3+掺杂对Lu2O3的立方相结构没有影响。在322 nm激发波长下发射出位于551 nm处Ho3+的5S2→5I8跃迁;在551 nm监测下,合成的Ho3+、Bi3+共掺杂Lu2O3荧光粉出现Bi3+的322 nm特征激发峰以及Ho3+的448 nm处的5I8→5F1跃迁。当Bi3+掺杂浓度为1.5%时,Bi3+对Ho3+的能量传递最有效,比单掺Ho3+样品发射强度提高了34.8倍。Lu98.5%−yO3:1.5%Ho3+, y%Bi3+(y=1,1.5,2)样品,随着Bi3+掺杂浓度增加,用980 nm激发比322 nm激发在551 nm处获得的光强分别提高了 13.3倍、16.8倍、14.2倍。通过计算得到Bi3+和Ho3+之间的能量传递临界距离为2.979 nm,且Bi3+与Ho3+之间的能量传递是通过偶极-四极相互作用实现的。Abstract: Bi3+ doped Lu1-xO3: x%Ho3+ metal ion phosphors were prepared using the high-temperature solid-phase method. The crystal structures of Bi3+ doped Lu1-xO3: x%Ho3+ phosphors, the Bi3+→Ho3+ energy transfer rule in Lu2O3 matrix and the luminescent properties of synthetic powders with different doping concentrations were investigated. X-ray diffraction results showed that Bi3+ and Ho3+ doping had no effect on the cubic phase structure of Lu2O3. Lu2O3: Ho3+, Bi3+ phosphor emitted 5S2→5I8 transition of Ho3+ at 551 nm under an excitation wavelength of 322 nm, and exhibited 1S0→3P1 characteristic transition of Bi3+ at 322 nm and 5I8→5F1 transition of Ho3+ at 448 nm under an emission wavelength of 551 nm. When the doping concentration of Bi3+ was 1.5%, the effect was most effective for the energy transfer of Ho3+, which increased by a factor of 34.8 compared to that of the single-doped Ho3+ sample. For Lu98.5%−yO3:1.5%Ho3+, y%Bi3+(y=1, 1.5, 2), with the increase of Bi3+ ions concentration, the luminescence intensity at 551 nm under 980-nm excitation increased by a factor of 13.3, 16.8 and 14.2, respectively, compared to that of under 322-nm excitation. The energy transfer critical distance between Bi3+ and Ho3+ was calculated to be 2.979 nm, and the energy transfer between Bi3+ and Ho3+ was achieved by dipole-quadrupole interaction.
-
Key words:
- phosphor /
- luminescence propertie /
- energy transfer /
- multipolar interaction
-
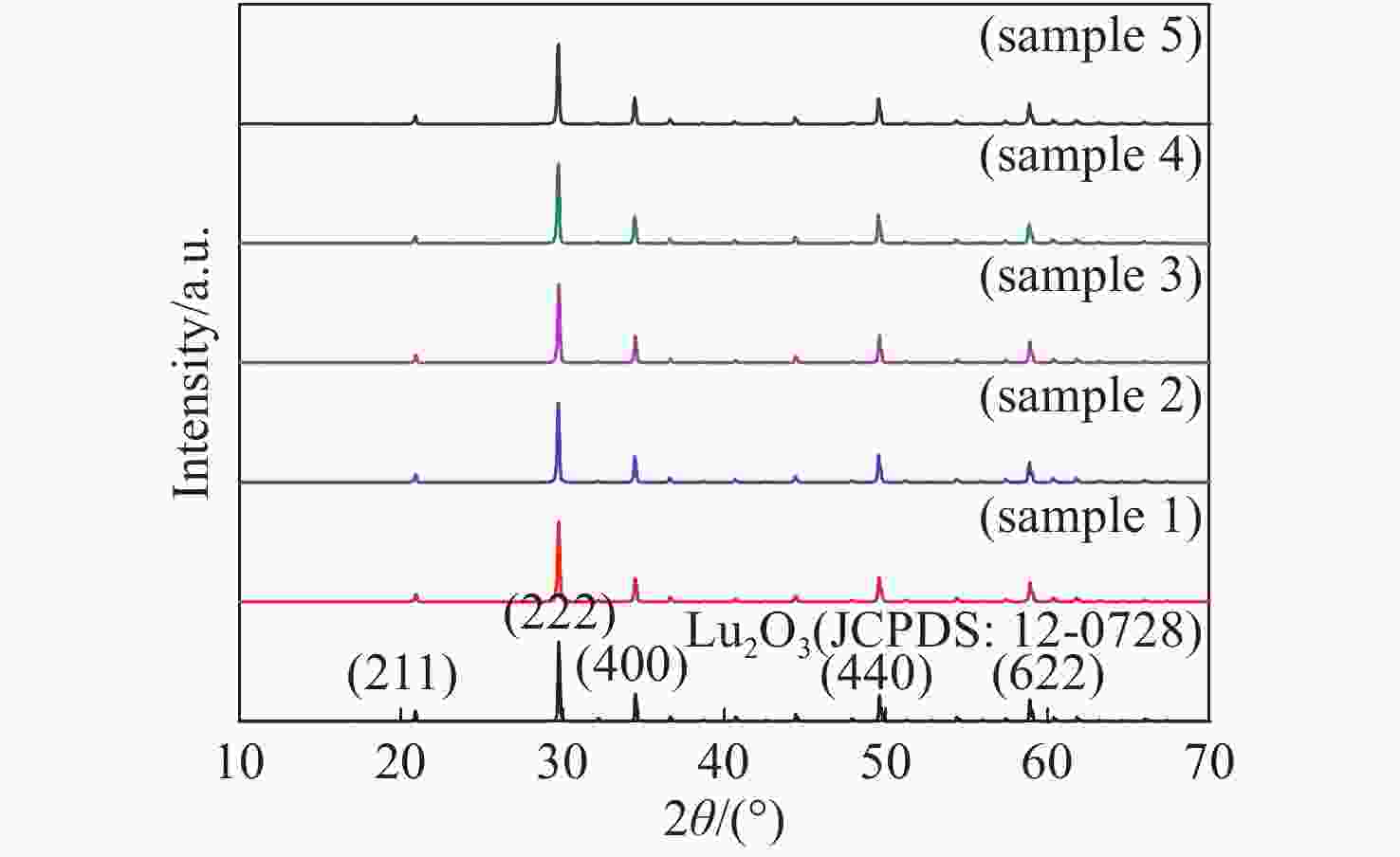
表 1 不同摩尔分数的Bi3+掺杂Lu2O3:Ho3+荧光粉
Table 1. Bi3+ doped Lu2O3:Ho3+ phosphors at different doping concentrations
sample Lu2O3 Ho3+ Bi3+ 1 98.5% 1.5% 0% 2 98.5% 0% 1.5% 3 97.5% 1.5% 1.0% 4 97.0% 1.5% 1.5% 5 96.5% 1.5% 2.0% -
[1] 贾玉涛. 稀土离子Ho3+掺杂氧化物上转换光致发光的研究[D]. 苏州: 苏州大学, 2010.JIA Y T. Study on up-conversion photo luminescence of rare earth ions Ho3+ doped oxides[D]. Suzhou: Soochow University, 2010. (in Chinese) [2] FENG L, WU Y S. Optical transitions of Ho3+ in oxyfluoride glasses and upconversion luminescence of Ho3+/Yb3+ -codoped oxyfluoride glasses[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2015, 142: 232-238. doi: 10.1016/j.saa.2015.01.066 [3] BHARGAVI K, RAO M S, SUDARSAN V, et al. Influence of Al3+ ions on self up-conversion in Ho3+ doped lead silicate glasses[J]. Optical Materials, 2014, 36(7): 1189-1196. doi: 10.1016/j.optmat.2014.02.027 [4] SURESH B, ZHYDACHEVSKII Y, BRIK M G, et al. Amplification of green emission of Ho3+ ions in lead silicate glasses by sensitizing with Bi3+ ions[J]. Journal of Alloys and Compounds, 2016, 683: 114-122. doi: 10.1016/j.jallcom.2016.05.056 [5] 李新跃, 邓建国, 刘东亮. 氧化物掺杂稀土的上转换材料研究进展[J]. 信阳师范学院学报: 自然科学版,2011,24(3):414-420.LI X Y, DENG J G, LIU D L. Research progress of upconversion materials of oxide matrix with doped rare earths[J]. Journal of Xinyang Normal University:Natural Science Edition, 2011, 24(3): 414-420. (in Chinese) [6] 李佳钰. Bi3+和几种稀土离子掺杂新型发光材料的合成及性质研究[D]. 沈阳: 沈阳师范大学, 2018.LI J Y. Study of the synthesis and luminescent properties of Bi3+ and several rare earth ions doped new optical materials[D]. Shenyang: Shenyang Normal University, 2018. (in Chinese). [7] 郭如旺, 郭常新. Lu2O3: Bi3+粉末晶体发光性能的研究[J]. 中国稀土学报,2007,25(5):533-539. doi: 10.3321/j.issn:1000-4343.2007.05.003GUO R W, GUO CH X. Luminescent properties of nano- and submicron-crystal Lu2O3: Bi3+[J]. Journal of the Chinese Rare Earth Society, 2007, 25(5): 533-539. (in Chinese) doi: 10.3321/j.issn:1000-4343.2007.05.003 [8] RAO T K V, KAMAL C S, SAMUEL T, et al. Color tunable luminescence from LaAlO3: Bi3+, Ho3+ doped phosphors for field emission displays[J]. Journal of Materials Science:Materials in Electronics, 2017, 29(2): 1011-1017. [9] XUE J P, WANG X F, JEONG J H, et al. Spectral and energy transfer in Bi3+–Ren+ (n = 2, 3, 4) co-doped phosphors: extended optical applications[J]. Physical Chemistry Chemical Physics, 2018, 20(17): 11516-11541. doi: 10.1039/C8CP00433A [10] ZENG L W, LIU Y, LIN B H, et al. Rational design of Bi3+/Ln3+: GdVO4 (Ln=Eu, Sm, Dy, Ho) nanophosphor: synthesis, characterization and color-tunable property[J]. Optical Materials, 2018, 77: 204-210. doi: 10.1016/j.optmat.2018.01.040 [11] 赵海琴, 王林香, 庹娟, 等. Li+/Bi3+掺杂Lu2O3: Ho3+荧光粉的制备及其发光特性[J]. 激光与光电子学进展,2018,55(8):081602.ZHAO H Q, WANG L X, TUO J, et al. Preparation and luminescent properties of Li+/Bi3+ co-doped Lu2O3: Ho3+ phosphors[J]. Laser &Optoelectronics Progress, 2018, 55(8): 081602. (in Chinese) [12] 肖全兰, 孟建新, 谢丽娟, 等. Bi3+掺杂对YVO4: Yb3+近红外发光的敏化作用[J]. 物理化学学报,2011,27(10):2427-2431. doi: 10.3866/PKU.WHXB20110928XIAO Q L, MENG J X, XIE L J, et al. Near-infrared luminescence enhancement by co-doped Bi3+ in YVO4: Yb3+[J]. Acta Physico-Chimica Sinica, 2011, 27(10): 2427-2431. (in Chinese) doi: 10.3866/PKU.WHXB20110928 [13] KHAN W, ZHOU L LIANG, et al. Luminescence enhancement and energy transfers of Ce3+ and Sm3+ in CaSrSiO4 phosphor[J]. Journal of Materials Chemistry C, 2018, 6(28): 7612-7618. doi: 10.1039/C8TC02143K [14] TAO ZH X, TSUBOI T, HUANG Y L, et al. Photoluminescence properties of Eu3+-doped glaserite-type orthovanadates CsK2Gd[VO4]2[J]. Inorganic Chemistry, 2014, 53(8): 4161-4168. doi: 10.1021/ic500208h [15] XIA ZH G, MIAO SH H, CHEN M Y, et al. Structure, crystallographic sites, and tunable luminescence properties of Eu2+ and Ce3+/Li+-activated Ca1.65Sr0.35SiO4 phosphors[J]. Inorganic Chemistry, 2015, 54(16): 7684-7691. doi: 10.1021/acs.inorgchem.5b00455 [16] GUO Q, WANG Q, JIANG L, et al. A novel apatite, Lu5(SiO4)3N: (Ce, Tb), phosphor material: synthesis, structure and applications for NUV-LEDs[J]. Physical Chemistry Chemical Physics, 2016, 18(23): 15545-15554. doi: 10.1039/C6CP01512C [17] 胡莲莲, 艾尔肯·斯地克, 万英, 等. Dy3+、Tm3+共掺杂Ca2MgSi2O7的发光特性[J]. 发光学报,2018,39(7):948-954. doi: 10.3788/fgxb20183907.0948HU L L, AIERKEN S, WAN Y, et al. Luminescent properties of Dy3+, Tm3+ co-doped Ca2MgSi2O7[J]. Chinese Journal of Luminescence, 2018, 39(7): 948-954. (in Chinese) doi: 10.3788/fgxb20183907.0948 [18] DEXTER D L, SCHULMAN J H. Theory of concentration quenching in inorganic phosphors[J]. Journal of Chemical Physics, 1954, 22(6): 1063-1070. doi: 10.1063/1.1740265 [19] ZHOU H P, JIN Y, JIANG M S, et al. A single-phased tunable emission phosphor MgY2Si3O10: Eu3+, Bi3+ with efficient energy transfer for white LEDs[J]. Dalton Transactions, 2015, 44(3): 1102-1109. doi: 10.1039/C4DT02114B [20] 杨国辉, 陈凯, 王小军, 等. 基质组成变化及电荷补偿对NaM4(VO4)3: Eu3+(M=Mg, Ca)荧光性能的调控[J]. 发光学报,2019,40(6):725-734. doi: 10.3788/fgxb20194006.0725YANG G H, CHEN K, WANG X J, et al. Controlling emissions of NaM4(VO4)3: Eu3+(M=Mg, Ca) phosphor by adjusting base composition and charge compensation[J]. Chinese Journal of Luminescence, 2019, 40(6): 725-734. (in Chinese) doi: 10.3788/fgxb20194006.0725 [21] 糜万鑫, 曹丽丽, 楚司祺, 等. 绿色荧光粉Sr3P4O13: Ce3+, Tb3+的发光特性及Ce3+→Tb3+能量传递机理[J]. 光学学报,2019,39(8):0816002. doi: 10.3788/AOS201939.0816002MI W X, CAO L L, CHU S Q, et al. Luminescence properties and energy transfer mechanism of Sr3P4O13: Ce3+, Tb3+ green phosphors[J]. Acta Optica Sinica, 2019, 39(8): 0816002. (in Chinese) doi: 10.3788/AOS201939.0816002 [22] 于汀, 高明燕, 宋岩, 等. Dy3+, Eu3+共掺的LiGd(MoO4)2单一相荧光粉的合成、发光及能量传递[J]. 无机化学学报,2018,34(5):857-863. doi: 10.11862/CJIC.2018.116YU T, GAO M Y, SONG Y, et al. Synthesis, luminescence and energy transfer of Dy3+ and Eu3+ co-doped LiGd(MoO4)2 single-phase phosphors[J]. Chinese Journal of Inorganic Chemistry, 2018, 34(5): 857-863. (in Chinese) doi: 10.11862/CJIC.2018.116 [23] 苏小娜, 万英, 周芷萱, 等. Na2CaSiO4: Sm3+, Eu3+荧光粉的发光特性和能量传递[J]. 物理学报,2017,66(23):230701. doi: 10.7498/aps.66.230701SU X N, WAN Y, ZHOU ZH X, et al. Luminescence properties and energy transfer of Na2CaSiO4: Sm3+, Eu3+ phosphor[J]. Acta Physica Sinica, 2017, 66(23): 230701. (in Chinese) doi: 10.7498/aps.66.230701 [24] BLASSE G. Energy transfer in oxidic phosphors[J]. Physics Letters A, 1968, 28(6): 444-445. doi: 10.1016/0375-9601(68)90486-6 [25] XIE A, YUAN X M, SHI Y, et al. Photoluminescence characteristics of energy transfer between Eu3+ and Bi3+ in LiEu1−xBix (WO4)0.5(MoO4)1.5[J]. Journal of the American Ceramic Society, 2009, 92(10): 2254-2258. doi: 10.1111/j.1551-2916.2009.03195.x [26] 陈彩花, 杨国辉, 梁利芳, 等. 溶胶凝胶法合成CaYAlO4: Mn4+红色荧光粉及其荧光性能研究[J]. 发光学报,2017,38(5):567-573. doi: 10.3788/fgxb20173805.0567CHEN C H, YANG G H, LIANG L F, et al. Luminescent properties of CaYAlO4: Mn4+ red phosphors prepared by sol-gel method[J]. Chinese Journal of Luminescence, 2017, 38(5): 567-573. (in Chinese) doi: 10.3788/fgxb20173805.0567 [27] ZHANG Y, GONG W T, YU J J, et al. Multi-color luminescence properties and energy transfer behaviour in host-sensitized CaWO4: Tb3+, Eu3+ phosphors[J]. RSC Advances, 2016, 6(37): 30886-30894. doi: 10.1039/C6RA01862A [28] REISFELD R, LIEBLICH-SOFFER N. Energy transfer from UO22+ to Sm3+ in phosphate glass[J]. Journal of Solid State Chemistry, 1979, 28(3): 391-395. doi: 10.1016/0022-4596(79)90090-2 [29] HUANG C H, CHEN T M. A novel single-composition trichromatic white-light Ca3Y(GaO)3(BO3)4: Ce3+, Mn2+, Tb3+ phosphor for UV-light emitting diodes[J]. The Journal of Physical Chemistry C, 2011, 115(5): 2349-2355. doi: 10.1021/jp107856d [30] JHA K, JAYASIMHADRI M. Effective sensitization of Eu3+ and energy transfer in Sm3+/Eu3+ co-doped ZPBT glasses for CuPc based solar cell and w-LED applications[J]. Journal of Luminescence, 2018, 194: 102-107. doi: 10.1016/j.jlumin.2017.09.049 [31] PAULOSE P I, JOSE G, THOMAS V, et al. Sensitized fluorescence of Ce3+/Mn2+ system in phosphate glass[J]. Journal of Physics and Chemistry of Solids, 2003, 64(5): 841-846. doi: 10.1016/S0022-3697(02)00416-X -





 下载:
下载: