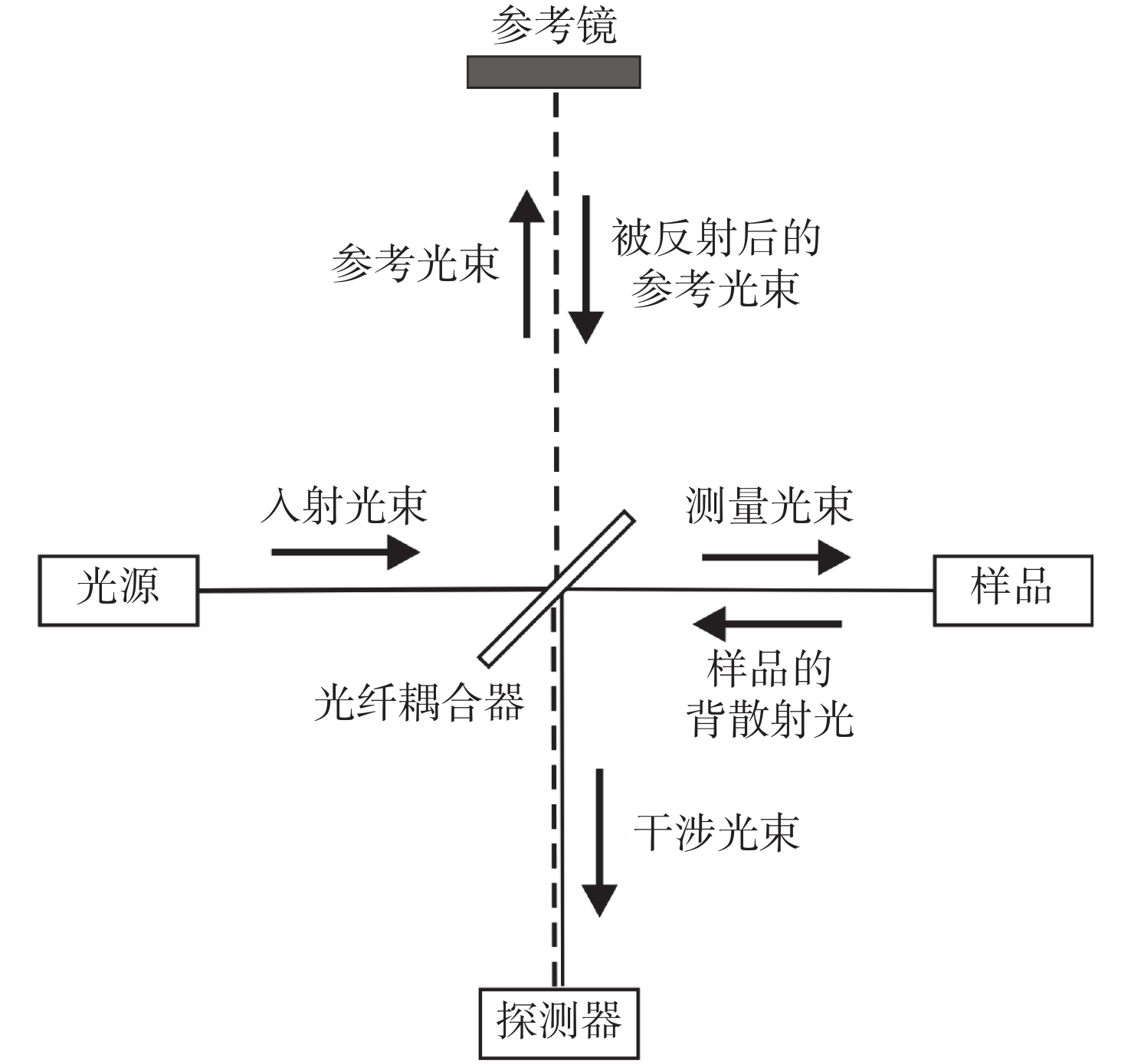
-
摘要: 光学相干层析成像(Optical Coherence Tomography,OCT)是一种基于低相干光干涉原理,利用样品背散/反射光与参考光相干的非接触非侵入性的新型成像技术,可提供具有微米级分辨率的一维深度,二维截面层析和三维立体的实时扫描图像。OCT技术具有非接触、无损伤、图像分辨率高且操作简单、便携等优点,主要应用于生物医学成像和诊断领域,弥补了共聚焦显微镜成像穿透深度低和超声波成像分辨率低的不足。目前,OCT技术已作为诊断视网膜疾病的临床标准,而且OCT技术结合内窥镜技术已成为临床上心血管及肠胃疾病诊断的重要工具,同时也为肌肉骨骼疾病,乃至癌症早期诊断、手术指导及术后康复提供依据。为了拓宽OCT技术的应用范围、提高医疗检测水平,研究人员正致力于增加OCT系统在生物组织中的穿透深度、提高系统的分辨率和信噪比、优化系统综合性能等方面的研究。本文论述了OCT系统的原理、分类,以及其在不同生物医学领域的应用及最新进展。Abstract: Optical Coherence Tomography (OCT) is a new imaging technique that uses interference in low coherent light by measuring the delay and magnitude of backscattered or reflected signals from the sample. OCT technology can provide real-time structural information with one-dimensional depth and two- and three-dimensional tomography at micron-scale resolution. Besides its high spatial resolution, OCT imaging is beneficial for its non-contact and non-invasive methodology. The system is also easy to operate and relatively portable. OCT technology is mainly applied in the biomedical imaging field for diagnoses, making up for the shortcomings of the low penetration depth in confocal microscopes and the low resolution in ultrasonic imaging. At present, OCT technology has been used as the clinical standard for the diagnosis of retinal diseases, and the combination of OCT technology and endoscope technology has become an important tool for the clinical diagnosis of cardiovascular and gastrointestinal diseases. It also provides references for early cancer diagnosis, surgical guidance and postoperative rehabilitation of musculoskeletal diseases. To broaden the application of OCT technology and improve its medical detection capabilities, researchers are committed to increasing the penetration depth of OCT imaging in biological tissue, improving the system's resolution and signal-to-noise ratio, and optimizing its overall performance. This review introduces the principle and classification of OCT systems, their applications and their recent progress in various biomedical fields.
-
Key words:
- optical coherence tomography /
- biomedical optics /
- infrared
-
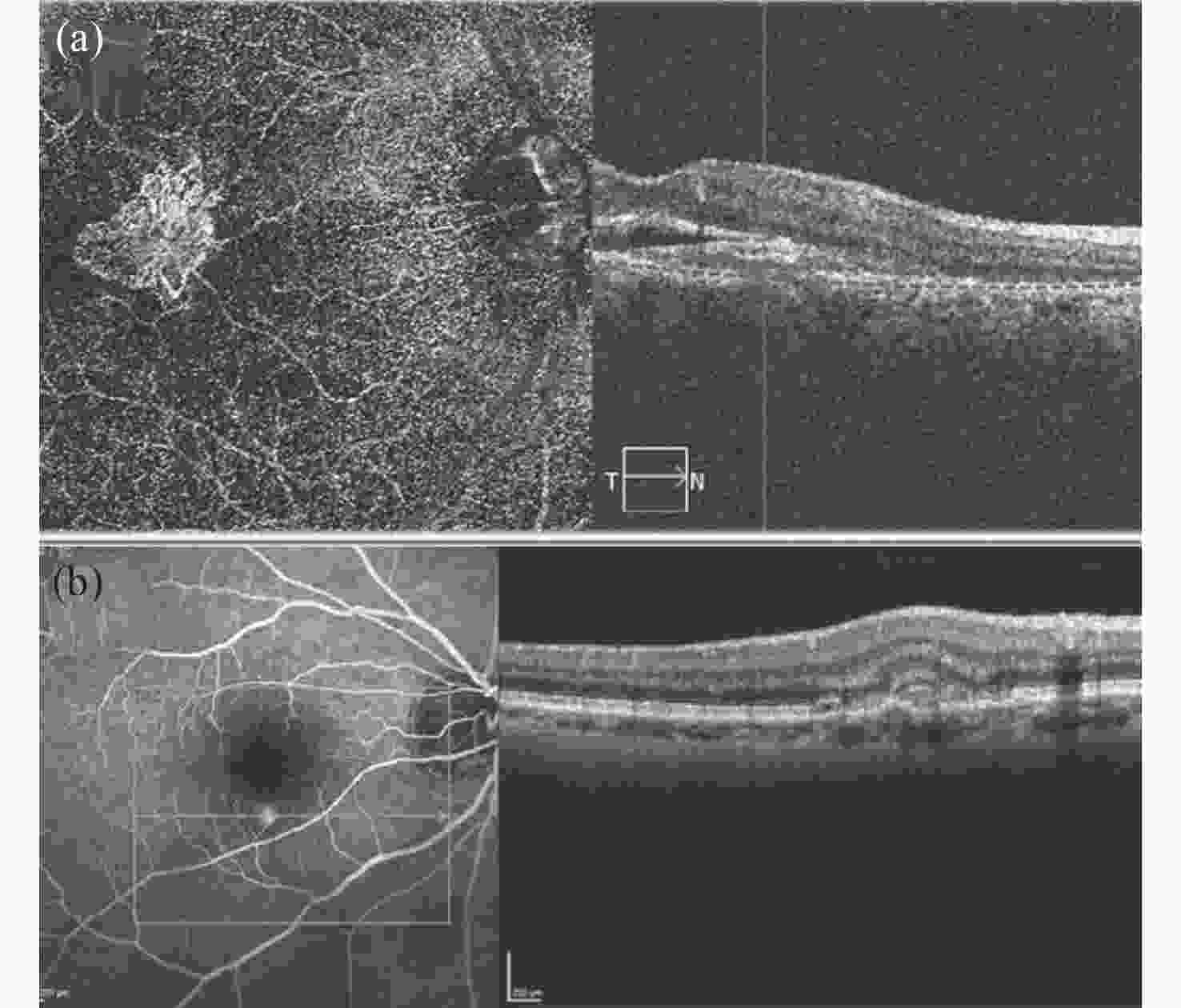
图 4 (a) 脉络膜新生血管的OCT血管造影照片。(b)在发生黄斑病变时的脉络膜新生血管的OCT照片[69]。
Figure 4. (a) OCT angiogram of choroidal neovascularization. (b) OCT photo of choroidal neovascularization during macular degeneration.
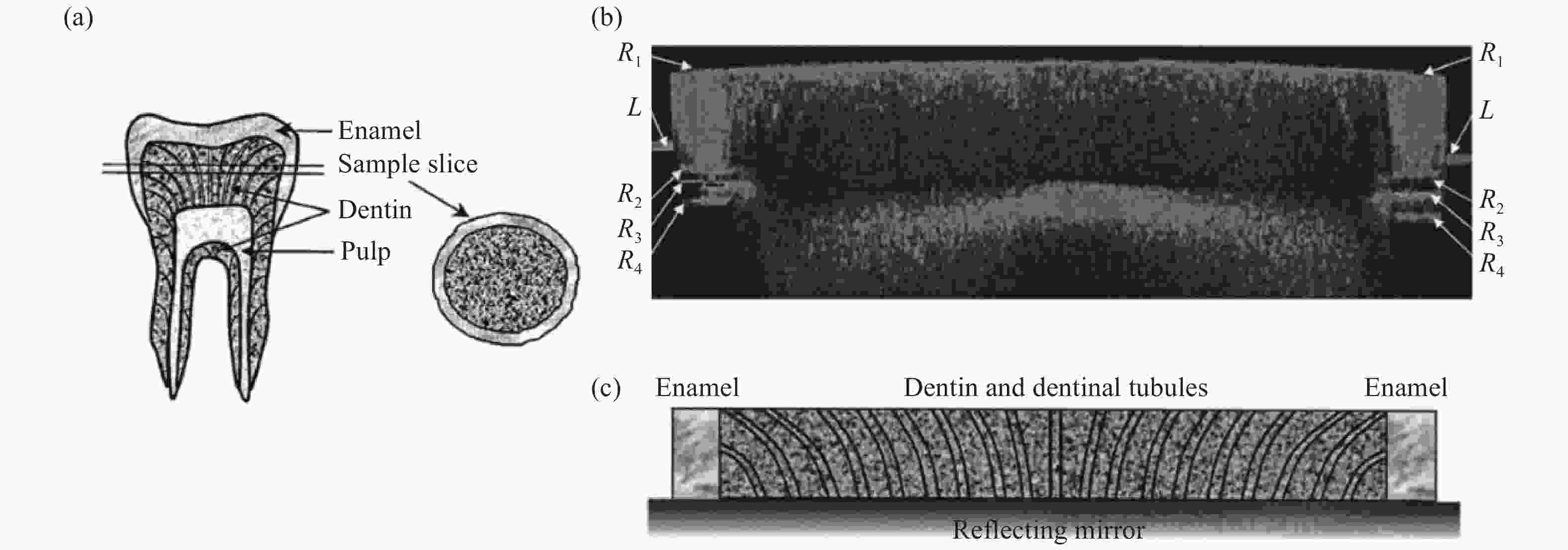
图 5 (a)人类臼齿矢状切面示意图(左)和平板切片样品(右)。(b)牙齿切片样品的偏振OCT照片(宽10.8 mm,深600 μm)。(c)与图(b)对应的齿形截面示意图[78]。
Figure 5. (a) Schematic diagram of a sagittal section of a human molar (left) and a slab sample (right). (b) PS-OCT image of a tooth sample extending through the diameter of the sample disk (10.8 mm wide by 600 μm deep). (c) Schematic diagram of a tooth’s cross section corresponding to the PS-OCT image in (b)[78].
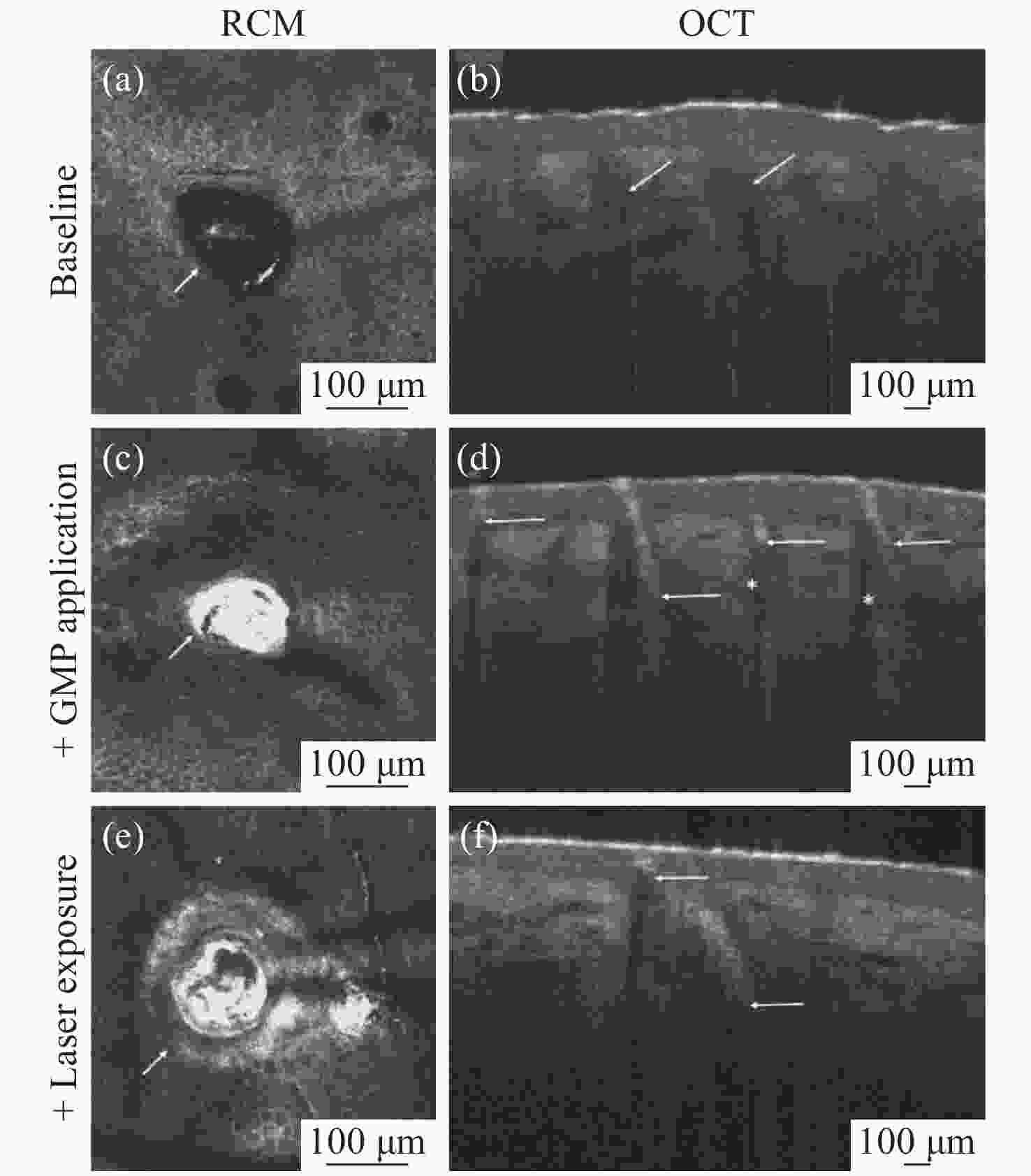
图 6 (a)无金微粒,(c)有金微粒和(e)激光照射有金微粒毛囊的反射共聚焦显微镜(RCM)图像。(b)无金微粒、(d)有金微粒和(f)激光照射有金微粒的毛囊的断面OCT图像[85]。
Figure 6. Reflectance Confocal Microscopy (RCM) image of a hair follicle (a) without gold microparticles (GMPs), (c) after the application of the GMPs and (e) after exposing the GMPs to a laser. OCT scan of a hair follicle (b) without GMPs, (d) after applying the GMPs, and (f) after exposing the GMPs to a laser[85].
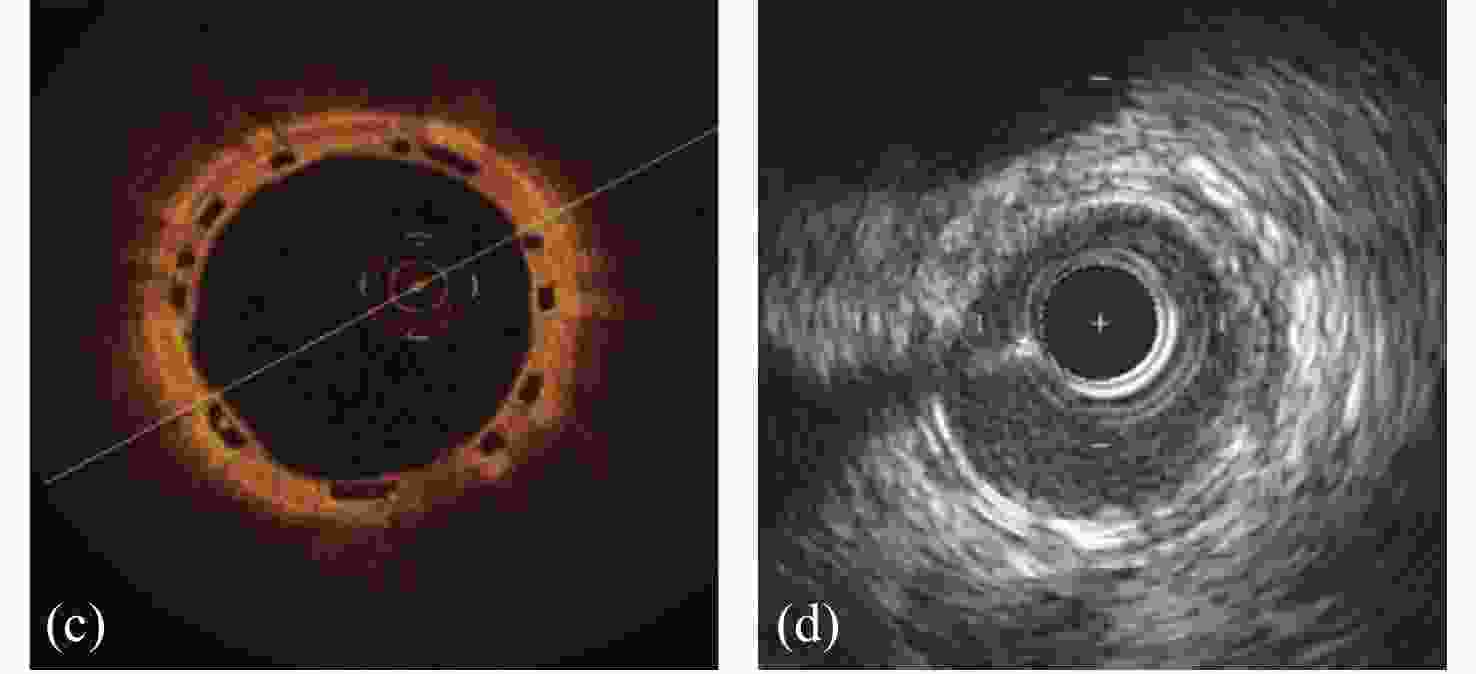
(a)在右侧冠状动脉内植入支架的血管造影图。(b)在右侧冠状动脉内植入的支架起作用后的血管造影图。(c)植入支架的血管的断面OCT照片,由于聚合物支架不反射光,因此呈现为清晰的(黑色)菱形。(d)植入支架的血管内超声照片,支架呈现为沿动脉壁周向分布的亮斑[98]。
(a) Angiogram of a stent implanted in the right coronary artery. (b) Angiogram of a worked stent implanted in the right coronary artery (c) Optical coherence tomography image of a blood vessel implanted with the stent. Due to their polymeric nature, struts of BRS do not reflect light and therefore appear as clear (black) rhomboids. (d) Intravascular ultrasound image of a blood vessel implanted with the stent. BRS struts are visualized as brighter foci distributed circumferentially around the arterial wall[98].
-
KALENDER W A. X-ray computed tomography[J]. Physics in Medicine and Biology, 2006, 51(13): R29-R43. doi: 10.1088/0031-9155/51/13/R03 CULJAT M O, GOLDENBERG D, TEWARI P, et al. A review of tissue substitutes for ultrasound imaging[J]. Ultrasound in Medicine and Biology, 2010, 36(6): 861-873. doi: 10.1016/j.ultrasmedbio.2010.02.012 IMAI K, MORI T, IZUMOTO H, et al. MR imaging-based localized intra-arterial thrombolysis assisted by mechanical clot disruption for acute ischemic stroke due to middle cerebral artery occlusion[J]. American Journal of Neuroradiology, 2011, 32(4): 748-752. doi: 10.3174/ajnr.A2353 NTZIACHRISTOS V, BREMER C, WEISSLEDER R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging[J]. European Radiology, 2003, 13(1): 195-208. doi: 10.1007/s00330-002-1524-x DIASPRO A, BIANCHINI P, VICIDOMINI G, et al. Multi-photon excitation microscopy[J]. Biomedical Engineering OnLine, 2006, 5(1): 36. doi: 10.1186/1475-925X-5-36 WEBB R H. Confocal optical microscopy[J]. Reports on Progress in Physics, 1996, 59(3): 427-471. doi: 10.1088/0034-4885/59/3/003 HUANG D, SWANSON E A, LIN C P, et al. Optical coherence tomography[J]. Science, 1991, 254(5035): 1178-1181. doi: 10.1126/science.1957169 FERCHER A F, HITZENBERGER C K, DREXLER W, et al. In vivo optical coherence tomography[J]. American Journal of Ophthalmology, 1993, 116(1): 113-114. doi: 10.1016/S0002-9394(14)71762-3 TOMLINS P H, WANG R K. Theory, developments and applications of optical coherence tomography[J]. Journal of Physics D:Applied Physics, 2005, 38(15): 2519-2535. doi: 10.1088/0022-3727/38/15/002 FERCHER A F, DREXLER W, HITZENBERGER C K, et al. Optical coherence tomography-principles and applications[J]. Reports on Progress in Physics, 2003, 66(2): 239-303. doi: 10.1088/0034-4885/66/2/204 FUJIMOTO J G, BREZINSKI M E, TEARNEY G J, et al. Optical biopsy and imaging using optical coherence tomography[J]. Nature Medicine, 1995, 1(9): 970-972. doi: 10.1038/nm0995-970 WELZEL J. Optical coherence tomography in dermatology: a review[J]. Skin Research and Technology, 2001, 7(1): 1-9. doi: 10.1034/j.1600-0846.2001.007001001.x JIVRAJ J, CHEN CH L, HUANG Y Z, et al. Smart laser osteotomy: Integrating a pulsed 1064nm fiber laser into the sample arm of a fiber optic 1310 nm oct system for ablation monitoring[J]. Biomedical Optics Express, 2018, 9(12): 6374-6387. doi: 10.1364/BOE.9.006374 RECHTMAN E, HARRIS A, KUMAR R, et al. An update on retinal circulation assessment technologies[J]. Current Eye Research, 2003, 27(6): 329-343. doi: 10.1076/ceyr.27.6.329.18193 SÁNCHEZ-GALEANA C A, BOWD C, ZANGWILL L M, et al. Short-wavelength automated perimetry results are correlated with optical coherence tomography retinal nerve fiber layer thickness measurements in glaucomatous eyes[J]. Ophthalmology, 2004, 111(10): 1866-1872. doi: 10.1016/j.ophtha.2004.04.017 SKOLARIKOS A. Differentiation between normal renal tissue and renal tumours using functional optical coherence tomography: a phase I in vivo human study[J]. BJU International, 2012, 110(8b): E421. doi: 10.1111/j.1464-410X.2012.11220.x TOMLINS P H, ADEGUN O K, HAGI-PAVLI E, et al. Scattering attenuation microscopy of oral epithelial dysplasia[J]. Journal of Biomedical Optics, 2010, 15(6): 066003. doi: 10.1117/1.3505019 ZHANG Q Q, WU X J, TANG T, et al. Quantitative analysis of rectal cancer by spectral domain optical coherence tomography[J]. Physics in Medicine and Biology, 2012, 57(16): 5235-5244. doi: 10.1088/0031-9155/57/16/5235 BAILEY T J, DAVIS D H, VANCE J E, et al. Spectral-domain optical coherence tomography as a noninvasive method to assess damaged and regenerating adult zebrafish retinas[J]. Investigative Ophthalmology &Visual Science, 2012, 53(6): 3126-3138. SCHWARTZ D M, FINGLER J, KIM D Y, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography[J]. Ophthalmology, 2014, 121(1): 180-187. doi: 10.1016/j.ophtha.2013.09.002 梁雨. 全光纤高速时域OCT系统研制[D]. 天津: 天津大学, 2010.LIANG Y. Development of all fiberhigh speed time-domain OCT system[D]. Tianjin: Tianjin University, 2010. (in Chinese) POVAZAY B, BIZHEVA K, UNTERHUBER A, et al. Submicrometer axial resolution optical coherence tomography[J]. Optics Letters, 2002, 27(20): 1800-1802. doi: 10.1364/OL.27.001800 BREZINSKI M E. Optical Coherence Tomography: Principles and Applications[M]. Amsterdam: Academic Press, 2006. POTSAID B, BAUMANN B, HUANG D, et al. Ultrahigh speed 1050nm swept source/fourier domain oct retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second[J]. Optics Express, 2010, 18(19): 20029-20048. doi: 10.1364/OE.18.020029 DREXLER W, MORGNER U, KÄRTNER F X, et al. In vivo ultrahigh-resolution optical coherence tomography[J]. Optics Letters, 1999, 24(17): 1221-1223. doi: 10.1364/OL.24.001221 SHEN K, LU H, BAIG S, et al. Improving lateral resolution and image quality of optical coherence tomography by the multi-frame superresolution technique for 3D tissue imaging[J]. Biomedical Optics Express, 2017, 8(11): 4887-4918. doi: 10.1364/BOE.8.004887 WANG B Q, LU R W, ZHANG Q X, et al. Breaking diffraction limit of lateral resolution in optical coherence tomography[J]. Quantitative Imaging in Medicine and Surgery, 2013, 3(5): 243-248. 李刚, 任钊, 林凌, 等. 高速线扫描OCT的可行性与光学成像特性的研究[J]. 中国生物医学工程学报,2007,26(1):89-93. doi: 10.3969/j.issn.0258-8021.2007.01.017LI G, REN ZH, LIN L, et al. Study on the feasibility and optical imaging properties of high speed line-focused OCT[J]. Chinese Journal of Biomedical Engineering, 2007, 26(1): 89-93. (in Chinese) doi: 10.3969/j.issn.0258-8021.2007.01.017 DICKENSHEETS D L, KINO G S. Silicon-micromachined scanning confocal optical microscope[J]. Journal of Microelectromechanical Systems, 1998, 7(1): 38-47. doi: 10.1109/84.661382 PAN Y, LANKENOU E, WELZEL J, et al. Optical coherence-gated imaging of biological tissues[J]. IEEE Journal of Selected Topics in Quantum Electronics, 1996, 2(4): 1029-1034. doi: 10.1109/2944.577332 SZYDLO J, DELACHENAL N, GIANOTTI R, et al. Air-turbine driven optical low-coherence reflectometry at 28.6-kHz scan repetition rate[J]. Optics Communications, 1998, 154(1-3): 1-4. doi: 10.1016/S0030-4018(98)00303-4 CAMPBELL D J, KRUG P A, FALCONER I S, et al. Rapid scan phase modulator for interferometric applications[J]. Applied Optics, 1981, 20(2): 335-342. doi: 10.1364/AO.20.000335 ROLLINS A M, KULKARNI M D, YAZDANFAR S, et al. In vivo video rate optical coherence tomography[J]. Optics Express, 1998, 3(6): 219-229. doi: 10.1364/OE.3.000219 DE BOER J F, CENSE B, PARK B H, et al. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography[J]. Optics Letters, 2003, 28(21): 2067-2069. doi: 10.1364/OL.28.002067 LEITGEB R, HITZENBERGER C K, FERCHER A F. Performance of fourier domain vs. time domain optical coherence tomography[J]. Optics Express, 2003, 11(8): 889-894. doi: 10.1364/OE.11.000889 AN L, LI P, SHEN T T, et al. High speed spectral domain optical coherence tomography for retinal imaging at 500,000 a-lines per second[J]. Biomedical Optics Express, 2011, 2(10): 2770-2783. doi: 10.1364/BOE.2.002770 LI P, AN L, LAN G P, et al. Extended imaging depth to 12 mm for 1050-nm spectral domain optical coherence tomography for imaging the whole anterior segment of the human eye at 120-khz a-scan rate[J]. Journal of Biomedical Optics, 2013, 18(1): 016012. doi: 10.1117/1.JBO.18.1.016012 GORA M, KARNOWSKI K, SZKULMOWSKI M, et al. Ultra high-speed swept source oct imaging of the anterior segment of human eye at 200 khz with adjustable imaging range[J]. Optics Express, 2009, 17(17): 14880-14894. doi: 10.1364/OE.17.014880 FERCHER A F, HITZENBERGER C K, KAMP G, et al. Measurement of intraocular distances by backscattering spectral interferometry[J]. Optics Communications, 1995, 117(1-2): 43-48. doi: 10.1016/0030-4018(95)00119-S WOJTKOWSKI M, LEITGEB R, KOWALCZYK A, et al. In vivo human retinal imaging by fourier domain optical coherence tomography[J]. Journal of Biomedical Optics, 2002, 7(3): 457-463. doi: 10.1117/1.1482379 WOJTKOWSKI M, BAJRASZEWSKI T, TARGOWSKI P, et al. Real-time in vivo ophthalmic imaging by ultrafast spectral optical coherence tomography[J]. Proceedings of SPIE, 2003: 4956. doi: 10.1117/12.477634 NASSIF N, CENSE B, PARK B H, et al. In vivo human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography[J]. Optics Letters, 2004, 29(5): 480-482. doi: 10.1364/OL.29.000480 POTSAID B, GORCZYNSKA I, SRINIVASAN V J, et al. Ultrahigh speed spectral/fourier domain oct ophthalmic imaging at 70,000 to 312,500 axial scans per second[J]. Optics Express, 2008, 16(19): 15149-15169. doi: 10.1364/OE.16.015149 SRINIVASAN V J, WOJTKOWSKI M, KO T H, et al. Intraretinal thickness mapping using three–dimensional, high–speed ultrahigh resolution oct[J]. Investigative Ophthalmology &Visual Science, 2005, 46(13): 1113. WOJTKOWSKI M, SRINIVASAN V, FUJIMOTO J G, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography[J]. Ophthalmology, 2005, 112(10): 1734-1746. doi: 10.1016/j.ophtha.2005.05.023 CHINN S R, SWANSON E A, FUJIMOTO J G. Optical coherence tomography using a frequency-tunable optical source[J]. Optics Letters, 1997, 22(5): 340-342. doi: 10.1364/OL.22.000340 HEE M R, HUANG D, SWANSON E A, et al. Polarization-sensitive low-coherence reflectometer for birefringence characterization and ranging[J]. Journal of the Optical Society of America B, 1992, 9(6): 903-908. doi: 10.1364/JOSAB.9.000903 WANG X J, MILNER T E, NELSON J S. Characterization of fluid flow velocity by optical doppler tomography[J]. Optics Letters, 1995, 20(11): 1337-1339. doi: 10.1364/OL.20.001337 MARIAMPILLAI A, STANDISH B A, MORIYAMA E H, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography[J]. Optics Letters, 2008, 33(13): 1530-1532. doi: 10.1364/OL.33.001530 WANG H, AL-QAISI M K, AKKIN T. Polarization-maintaining fiber based polarization-sensitive optical coherence tomography in spectral domain[J]. Optics Letters, 2010, 35(2): 154-156. doi: 10.1364/OL.35.000154 PARK B H, SAXER C E, SRINIVAS S M, et al. In vivo burn depth determination by high-speed fiber-based polarization sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2001, 6(4): 474-479. doi: 10.1117/1.1413208 FRIED D, XIE J, SHAFI S, et al. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2002, 7(4): 618-627. doi: 10.1117/1.1509752 ZHOU Q, KNIGHTON R W. Light scattering and form birefringence of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer[J]. Applied Optics, 1997, 36(10): 2273-2285. doi: 10.1364/AO.36.002273 WANG X J, MILNER T E, CHEN ZH P, et al. Measurement of fluid-flow-velocity profile in turbid media by the use of optical doppler tomography[J]. Applied Optics, 1997, 36(1): 144-149. doi: 10.1364/AO.36.000144 CHEN ZH P, MILNER T E, SRINIVAS S, et al. Noninvasive imaging of in vivo blood flow velocity using optical doppler tomography[J]. Optics Letters, 1997, 22(14): 1119-1121. doi: 10.1364/OL.22.001119 ALEXANDER L, CHOATE W. Rewriting the standard of care in diagnosis, management and intervention assessment[J]. Review of Optometry, 2004, 141(9): 1CE +. MUSCAT S, PARKS S, KEMP E, et al. Repeatability and reproducibility of macular thickness measurements with the humphrey oct system[J]. Investigative Ophthalmology &Visual Science, 2002, 43(2): 490-495. BOWD C, ZANGWILL L M, BLUMENTHAL E Z, et al. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender[J]. Journal of the Optical Society of America A, 2002, 19(1): 197-207. doi: 10.1364/JOSAA.19.000197 DE CARLO T E, ROMANO A, WAHEED N K, et al. A review of optical coherence tomography angiography (OCTA)[J]. International Journal of Retina and Vitreous, 2015, 1(1): 5. doi: 10.1186/s40942-015-0005-8 ZHANG Q Q, HUANG Y P, ZHANG T, et al. Wide-field imaging of retinal vasculature using optical coherence tomography-based microangiography provided by motion tracking[J]. Journal of Biomedical Optics, 2015, 20(6): 066008. doi: 10.1117/1.JBO.20.6.066008 WANG R K, AN L, FRANCIS P, et al. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography[J]. Optics Letters, 2010, 35(9): 1467-1469. doi: 10.1364/OL.35.001467 LI P, AN L, REIF R, et al. In vivo microstructural and microvascular imaging of the human corneo-scleral limbus using optical coherence tomography[J]. Biomedical Optics Express, 2011, 2(11): 3109-3118. doi: 10.1364/BOE.2.003109 ENFIELD J, JONATHAN E, LEAHY M. In vivo imaging of the microcirculation of the volar forearm using correlation mapping optical coherence tomography (cmOCT)[J]. Biomedical Optics Express, 2011, 2(5): 1184-1193. doi: 10.1364/BOE.2.001184 VAKOC B J, LANNING R M, TYRRELL J A, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging[J]. Nature Medicine, 2009, 15(10): 1219-1223. doi: 10.1038/nm.1971 TANNO N, KISHI S. Optical coherence tomographic imaging and clinical diagnosis[J]. Medical Imaging Technology, 1999, 17: 3-10. SCHUMAN J S, PULIAFITO C A, FUJIMOTO J G, et al.. Optical Coherence Tomography of Ocular Diseases[M]. 2nd ed. Thorofare, NJ: SLACK, New Jersey, 2004. FANG L Y, CUNEFARE D, WANG CH, et al. Automatic segmentation of nine retinal layer boundaries in oct images of non-exudative amd patients using deep learning and graph search[J]. Biomedical Optics Express, 2017, 8(5): 2732-2744. doi: 10.1364/BOE.8.002732 DE OLIVEIRA DIAS J R, ZHANG Q Q, GARCIA J M B, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source oct angiography[J]. Ophthalmology, 2018, 125(2): 255-266. doi: 10.1016/j.ophtha.2017.08.030 BOUTELEUX V, KODJIKIAN L, MENDES M, et al. Increased choroidal thickness: a new feature to monitor age-related macular degeneration recurrence[J]. Graefe’s Archive for Clinical and Experimental Ophthalmology, 2019, 257(4): 699-707. doi: 10.1007/s00417-018-04216-8 WU J H, SEBASTIAN R T, CHU C J, et al. Reduced macular vessel density and capillary perfusion in glaucoma detected using OCT angiography[J]. Current Eye Research, 2019, 44(5): 533-540. doi: 10.1080/02713683.2018.1563195 WANG J, HORMEL T T, YOU Q SH, et al. Robust non-perfusion area detection in three retinal plexuses using convolutional neural network in OCT angiography[J]. Biomedical Optics Express, 2020, 11(1): 330-345. doi: 10.1364/BOE.11.000330 CHAN V T T, SUN Z H, TANG SH M, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis[J]. Ophthalmology, 2019, 126(4): 497-510. doi: 10.1016/j.ophtha.2018.08.009 KAISER P K, BLODI B A, SHAPIRO H, et al. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration[J]. Ophthalmology, 2007, 114(10): 1868-1875. doi: 10.1016/j.ophtha.2007.04.030 APOSTOLOPOULOS M N, KOUTSANDREA C N, MOSCHOS M N, et al. Evaluation of successful macular hole surgery by optical coherence tomography and multifocal electroretinography[J]. American Journal of Ophthalmology, 2002, 134(5): 667-674. doi: 10.1016/S0002-9394(02)01700-2 TAN O, LI G, LU A T H, et al. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis[J]. Ophthalmology, 2008, 115(6): 949-956. doi: 10.1016/j.ophtha.2007.08.011 FUJIMOTO J, SWANSON E. The development, commercialization, and impact of optical coherence tomography[J]. Investigative Ophthalmology &Visual Science, 2016, 57(9): OCT1-OCT13. COLSTON B W, SATHYAM U S, DASILVA L B, et al. Dental OCT[J]. Optics Express, 1998, 3(6): 230-238. doi: 10.1364/OE.3.000230 WANG X J, MILNER T E, DE BOER J F, et al. Characterization of dentin and enamel by use of optical coherence tomography[J]. Applied Optics, 1999, 38(10): 2092-2096. doi: 10.1364/AO.38.002092 ADEN A, ANDERSON P, BURNETT G R, et al. Longitudinal correlation of 3D OCT to detect early stage erosion in bovine enamel[J]. Biomedical Optics Express, 2017, 8(2): 954-973. doi: 10.1364/BOE.8.000954 ZHOU Y, SHIMADA Y, MATIN K, et al. Assessment of root caries under wet and dry conditions using swept-source optical coherence tomography (SS-OCT)[J]. Dental Materials Journal, 2018, 37(6): 880-888. doi: 10.4012/dmj.2017-273 ALGHILAN M A, LIPPERT F, PLATT J A, et al. Impact of surface micromorphology and demineralization severity on enamel loss measurements by cross-polarization optical coherence tomography[J]. Journal of Dentistry, 2019, 81: 52-58. doi: 10.1016/j.jdent.2018.12.009 KUMAR ARAVETI S, HIRAISHI N, KOMINAMI N, et al. Swept-source optical coherence tomographic observation on prevalence and variations of cemento-enamel junction morphology[J]. Lasers in Medical Science, 2020, 35(1): 213-219. doi: 10.1007/s10103-019-02847-9 GAMBICHLER T, JAEDICKE V, TERRAS S. Optical coherence tomography in dermatology: technical and clinical aspects[J]. Archives of Dermatological Research, 2011, 303(7): 457-473. doi: 10.1007/s00403-011-1152-x SATTLER E C, KÄSTLE R, WELZEL J. Optical coherence tomography in dermatology[J]. Journal of Biomedical Optics, 2013, 18(6): 061224. doi: 10.1117/1.JBO.18.6.061224 FUCHS C S K, ORTNER V K, MOGENSEN M, et al. Transfollicular delivery of gold microparticles in healthy skin and acne vulgaris, assessed by in vivo reflectance confocal microscopy and optical coherence tomography[J]. Lasers in Surgery and Medicine, 2019, 51(5): 430-438. doi: 10.1002/lsm.23076 MOGENSEN M, THRANE L, JØRGENSEN T M, et al. OCT imaging of skin cancer and other dermatological diseases[J]. Journal of Biophotonics, 2009, 2(6-7): 442-451. doi: 10.1002/jbio.200910020 MOGENSEN M, NÜRNBERG B M, FORMAN J L, et al. In vivo thickness measurement of basal cell carcinoma and actinic keratosis with optical coherence tomography and 20-MHz ultrasound[J]. British Journal of Dermatology, 2009, 160(5): 1026-1033. doi: 10.1111/j.1365-2133.2008.09003.x KRATKIEWICZ K, MANWAR R, RAJABI-ESTARABADI A, et al. Photoacoustic/ultrasound/optical coherence tomography evaluation of melanoma lesion and healthy skin in a swine model[J]. Sensors, 2019, 19(12): 2815. doi: 10.3390/s19122815 ZHAO Y, CHU K K, WAX A. Enhanced depth penetration by dual-axis optical coherence tomography[J]. Proceedings of SPIE, 2019, 10867: 1086704. YOW A P, SRIVASTAVA R, CHENG J, et al.. Techniques and Applications in Skin OCT Analysis[M]. Springer, 2020. AKASAKA T, KUBO T, MIZUKOSHI M, et al. Pathophysiology of acute coronary syndrome assessed by optical coherence tomography[J]. Journal of Cardiology, 2010, 56(1): 8-14. doi: 10.1016/j.jjcc.2010.05.005 COSTOPOULOS C, BROWN A J, TENG ZH ZH, et al. Intravascular ultrasound and optical coherence tomography imaging of coronary atherosclerosis[J]. International Journal of Cardiovascular Imaging, 2016, 32(1): 189-200. doi: 10.1007/s10554-015-0701-3 SUN R, SUN L P, FU Y D, et al. Culprit plaque characteristics in women vs men with a first ST-segment elevation myocardial infarction: in vivo optical coherence tomography insights[J]. Clinical Cardiology, 2017, 40(12): 1285-1290. doi: 10.1002/clc.22825 LIANG Y T, LIU L W, HU S Y, et al. Characterizing physical properties and in vivo OCT imaging study of Cu-Sn-S nanocrystals[J]. AIP Advances, 2017, 7(1): 015012. doi: 10.1063/1.4973731 YANG SH ZH, CHEN H B, LIU L W, et al. OCT imaging detection of brain blood vessels in mouse, based on semiconducting polymer nanoparticles[J]. Analyst, 2017, 142(23): 4503-4510. doi: 10.1039/C7AN01245D LIU L W, HU S Y, WANG Y, et al. Optimizing the synthesis of core/shell structure Au@Cu2S nanocrystals as contrast-enhanced for bioimaging detection[J]. Scientific Reports, 2018, 8(1): 8866. doi: 10.1038/s41598-018-27015-x MURATA A, WALLACE-BRADLEY D, TELLEZ A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation[J]. JACC:Cardiovascular Imaging, 2010, 3(1): 76-84. doi: 10.1016/j.jcmg.2009.09.018 PERKINS L E L, RIPPY M K. Balloons and stents and scaffolds: preclinical evaluation of interventional devices for occlusive arterial disease[J]. Toxicologic Pathology, 2019, 47(3): 297-310. doi: 10.1177/0192623318815604 LIU X L, SUN CH B, TIAN J T, et al. Shrinkage as a potential mechanism of recurrent events in patients with a large vulnerable plaque[J]. Journal of Cardiovascular Medicine, 2019, 20(8): 518-524. doi: 10.2459/JCM.0000000000000783 WANG J Q, PARITALA P K, MENDIETA J B, et al. Optical coherence tomography-based patient-specific coronary artery reconstruction and fluid–structure interaction simulation[J]. Biomechanics and Modeling in Mechanobiology, 2020, 19(1): 7-20. doi: 10.1007/s10237-019-01191-9 NAKAMURA T, HORIKOSHI T, KUGIYAMA K. Relationship of a thinned medial layer to the attenuated contractile response in atherosclerotic coronary arteries[J]. American Journal of Physiology-Heart and Circulatory Physiology, 2020, 318(1): H135-H142. doi: 10.1152/ajpheart.00537.2019 KHOLODNYKH A I, PETROVA I Y, LARIN K V, et al. Precision of measurement of tissue optical properties with optical coherence tomography[J]. Applied Optics, 2003, 42(16): 3027-3037. doi: 10.1364/AO.42.003027 SONG Y CH, GARCIA S, FROMETA Y, et al. Quantitative assessment of hemodynamic and structural characteristics of in vivo brain tissue using total diffuse reflectance spectrum measured in a non-contact fashion[J]. Biomedical Optics Express, 2017, 8(1): 78-103. doi: 10.1364/BOE.8.000078 SU Y, YAO X S, LI ZH H, et al. Measurements of the thermal coefficient of optical attenuation at different depth regions of in vivo human skins using optical coherence tomography: a pilot study[J]. Biomedical Optics Express, 2015, 6(2): 500-513. doi: 10.1364/BOE.6.000500 MEI L, SOMESFALEAN G, SVANBERG S. Frequency-modulated light scattering interferometry employed for optical properties and dynamics studies of turbid media[J]. Biomedical Optics Express, 2014, 5(8): 2810-2822. doi: 10.1364/BOE.5.002810 KHOLODNYKH A I, PETROVA I Y, MOTAMEDI M, et al. Accurate measurement of total attenuation coefficient of thin tissue with optical coherence tomography[J]. IEEE Journal of Selected Topics in Quantum Electronics, 2003, 9(2): 210-221. doi: 10.1109/JSTQE.2003.814194 LI K Y, LIANG W X, YANG Z H, et al. Robust, accurate depth-resolved attenuation characterization in optical coherence tomography[J]. Biomedical Optics Express, 2020, 11(2): 672-687. doi: 10.1364/BOE.382493 LEVITZ D, THRANE L, FROSZ M H, et al. Determination of optical scattering properties of highly-scattering media in optical coherence tomography images[J]. Optics Express, 2004, 12(2): 249-259. doi: 10.1364/OPEX.12.000249 SORIN W V, GRAY D F. Simultaneous thickness and group index measurement using optical low-coherence reflectometry[J]. IEEE Photonics Technology Letters, 1992, 4(1): 105-107. doi: 10.1109/68.124892 WANG X J, MILNER T E, DHOND R P, et al. Characterization of human scalp hairs by optical low-coherence reflectometry[J]. Optics Letters, 1995, 20(6): 524-526. doi: 10.1364/OL.20.000524 WANG X J, MILNER T E, CHANG M C, et al. Group refractive index measurement of dry and hydrated type I collagen films using optical low-coherence reflectometry[J]. Journal of Biomedical Optics, 1996, 1(2): 212-216. doi: 10.1117/12.227699 ZVYAGIN A V, SILVA K K M B D, ALEXANDROV S A, et al. Refractive index tomography of turbid media by bifocal optical coherence refractometry[J]. Optics Express, 2003, 11(25): 3503-3517. doi: 10.1364/OE.11.003503 -





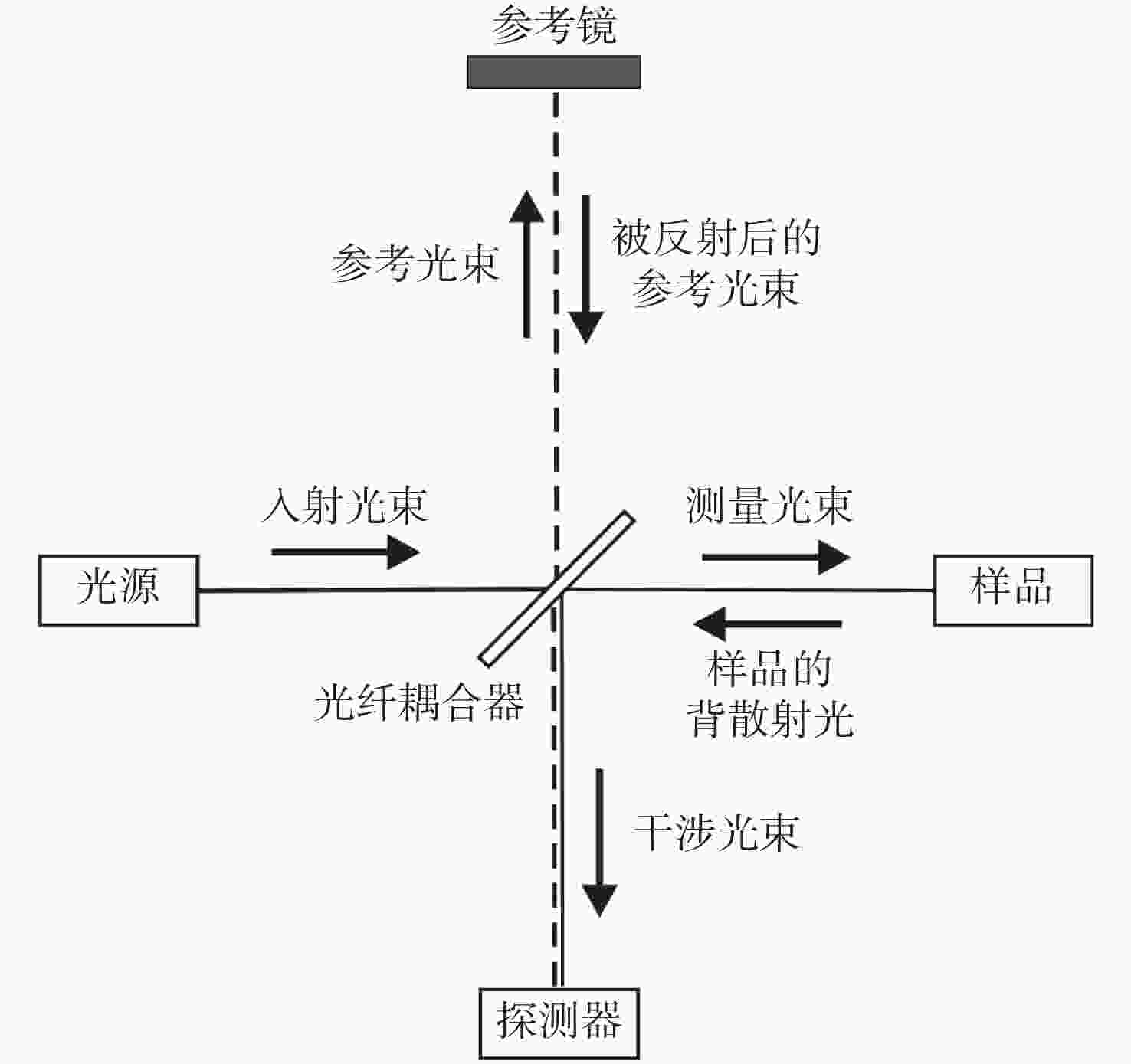
 下载:
下载: