Photothermal properties of gold nanostars therapeutic agent and its application in photothermal therapy and optical coherence tomography
-
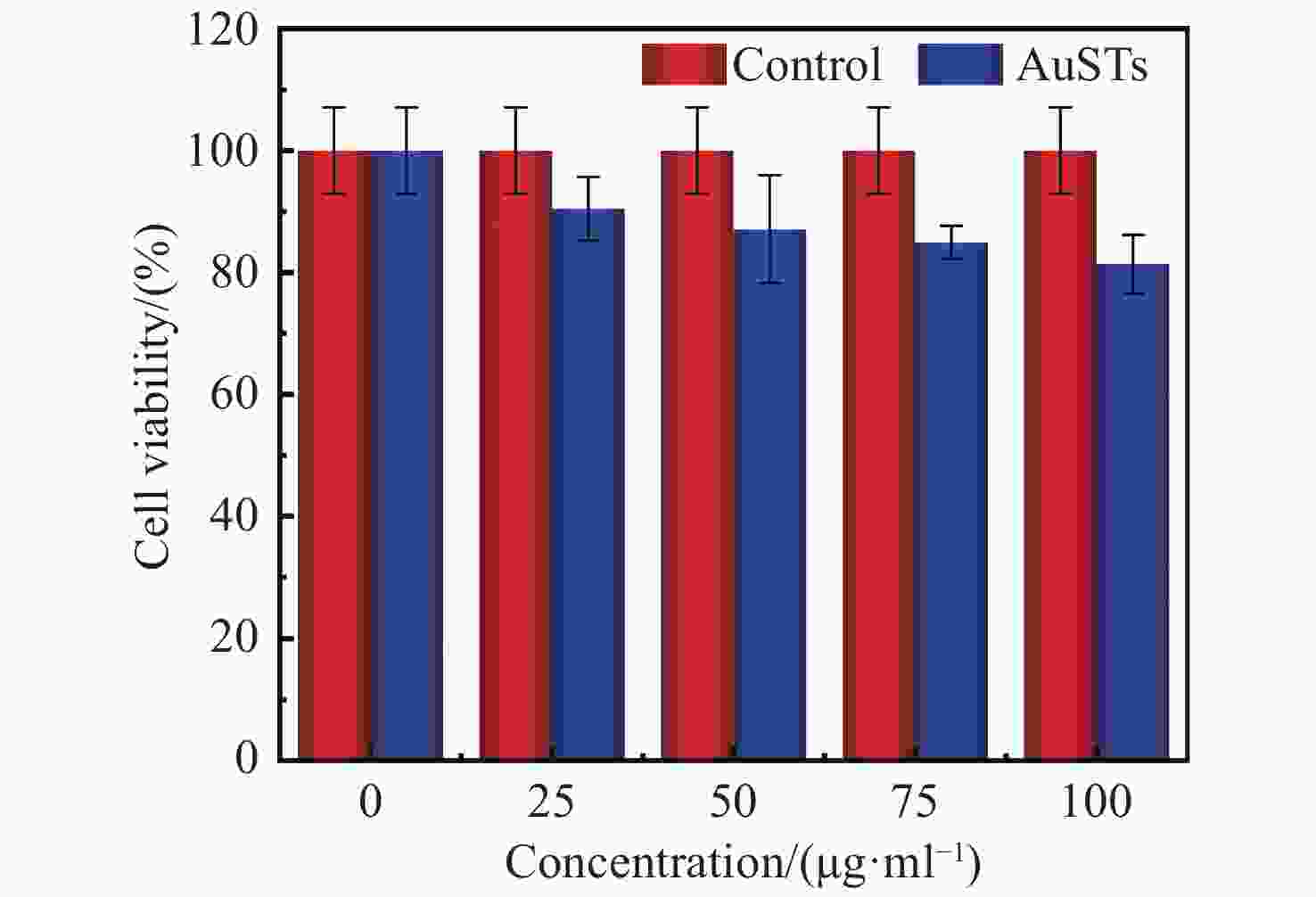
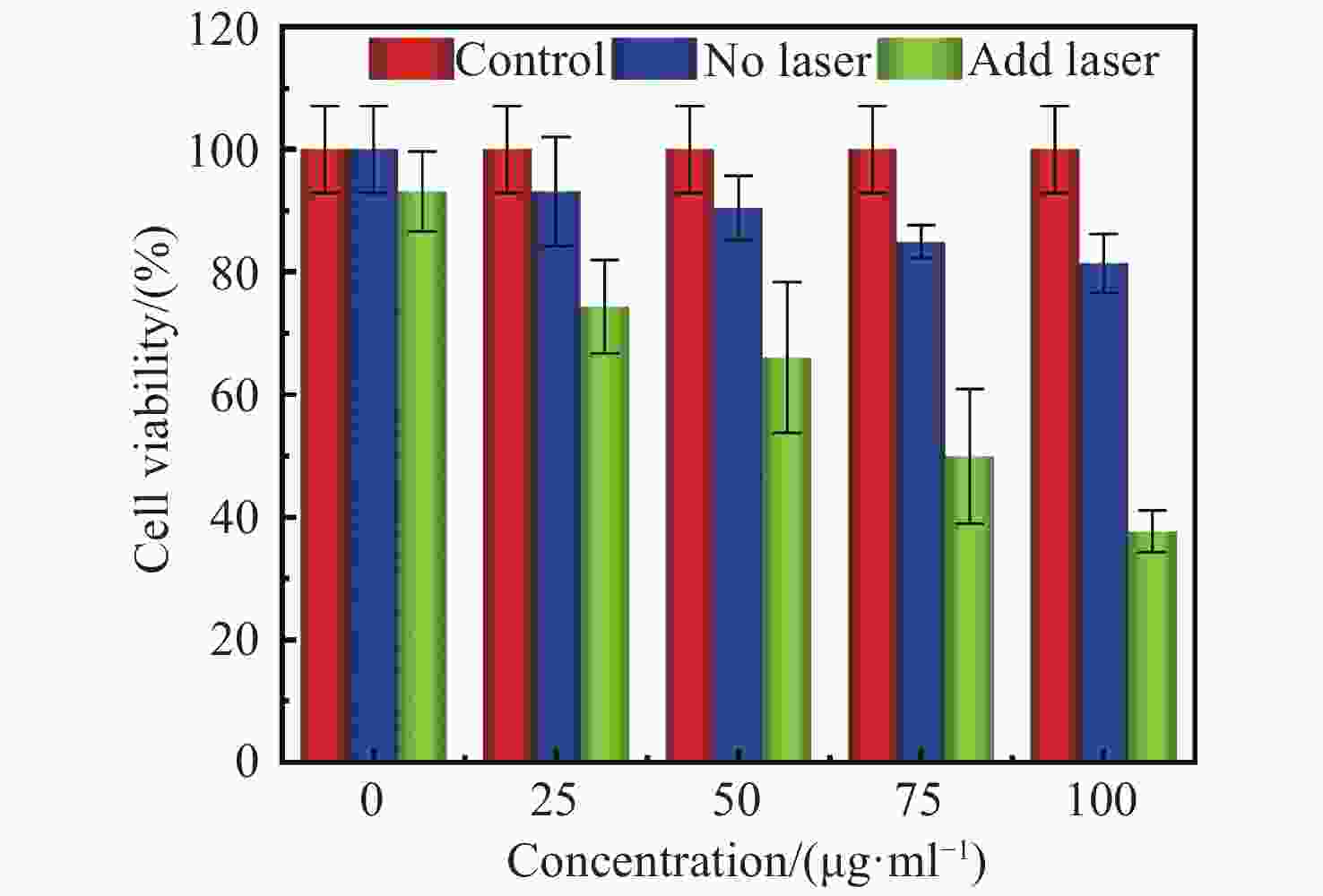
摘要: 为了开发一种优异的用于光热治疗和光学相干层析成像的金纳米星诊疗剂,对金纳米星的制备、光热特性以及光热治疗和光学相干层析成像中的应用进行研究。利用尖端结构增强金纳米材料的局域表面等离子体共振特性,通过种子介导法制备了多枝化的金纳米星,多尖端的结构使其具有明显的光热效果,并探究了其作为光热治疗的诊疗剂和光学相干层析成像造影剂的效果。实验结果表明:多枝化的金纳米星诊疗剂相比于金纳米粒子具有较高的光热转换效率,达到42%;具有较好的生物兼容性,在100 μg/mL浓度下,人乳腺癌细胞存活率为82%;而且具有较好的癌细胞光热治疗效果,在100 μg/mL浓度下,经激光照射后,人乳腺癌细胞被有效杀死,其存活率降至37%;同时,金纳米星诊疗剂还具有较好的光学相干层析成像造影效果,可显著提高信号强度和造影深度。金纳米星诊疗剂既具有高效光热治疗能力,又具备优异光学相干层析成像造影成像能力,是一种非常有前景的多功能诊疗剂。Abstract: In order to develop an excellent Au nanostars therapeutic agent for photothermal therapy and optical coherence tomography, we research the preparation of gold nanostars, photothermal properties, photothermal therapy and applications in optical coherence tomography. By adopting the tip structure to enhance the local surface plasmon resonance properties of gold nanomaterials, the multi-branched Au nanostars is prepared by seed mediated method. The multi-tip structure enables the Au nanostars to have obvious photothermal effect, then its effect as a therapeutic agent for photothermal therapy and contrast agent for optical coherence tomography is explored. The experimental results showed that compared with Au nanoparticles, the multi-branched Au nanostars had a higher photothermal conversion efficiency of 42%, and has good biocompatibility. At the concentration of 100 μg/mL, the survival rate of human breast cancer cells is 82%. Human breast cancer cells are effectively killed by laser irradiation at the concentration of 100 μg/mL, and the survival rate is significantly reduced to 37%. At the same time, Au nanostars also has better optical coherence tomography imaging effect, significantly improving the signal intensity and imaging depth. Au nanostars is a promising multifunctional therapeutic agent with both efficient photothermal therapy and excellent optical coherence tomography imaging capability.
-
Key words:
- gold nanostars /
- photothermal therapy /
- optical coherence tomography
-
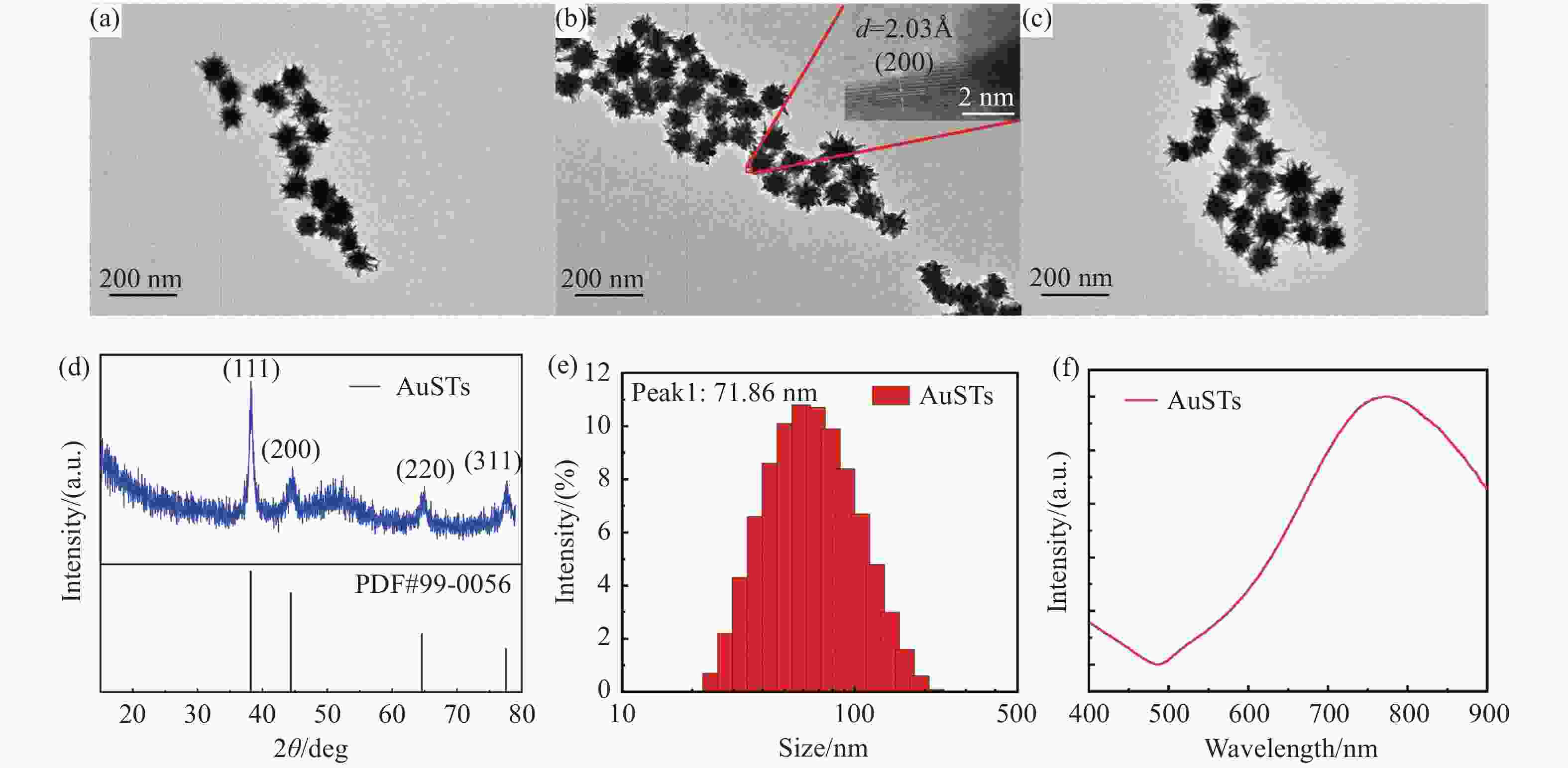
图 1 金纳米星的表征。Ag+浓度分别为(a)1 mM,(b)2 mM和(c)4 mM金纳米星的透射电子显微镜照片。(d)金纳米星的XRD图谱。(e)金纳米星的粒径分析。(f)金纳米星的吸收光谱
Figure 1. Characterization of AuSTs. Transmission electron micrographs of gold nanostars with Ag+ concentrations of (a) 1 mM, (b) 2 mM and (c) 4 mM. (d) XRD patterns of AuSTs. (e) Particle size of AuSTs. (f) Absorption spectra of AuSTs
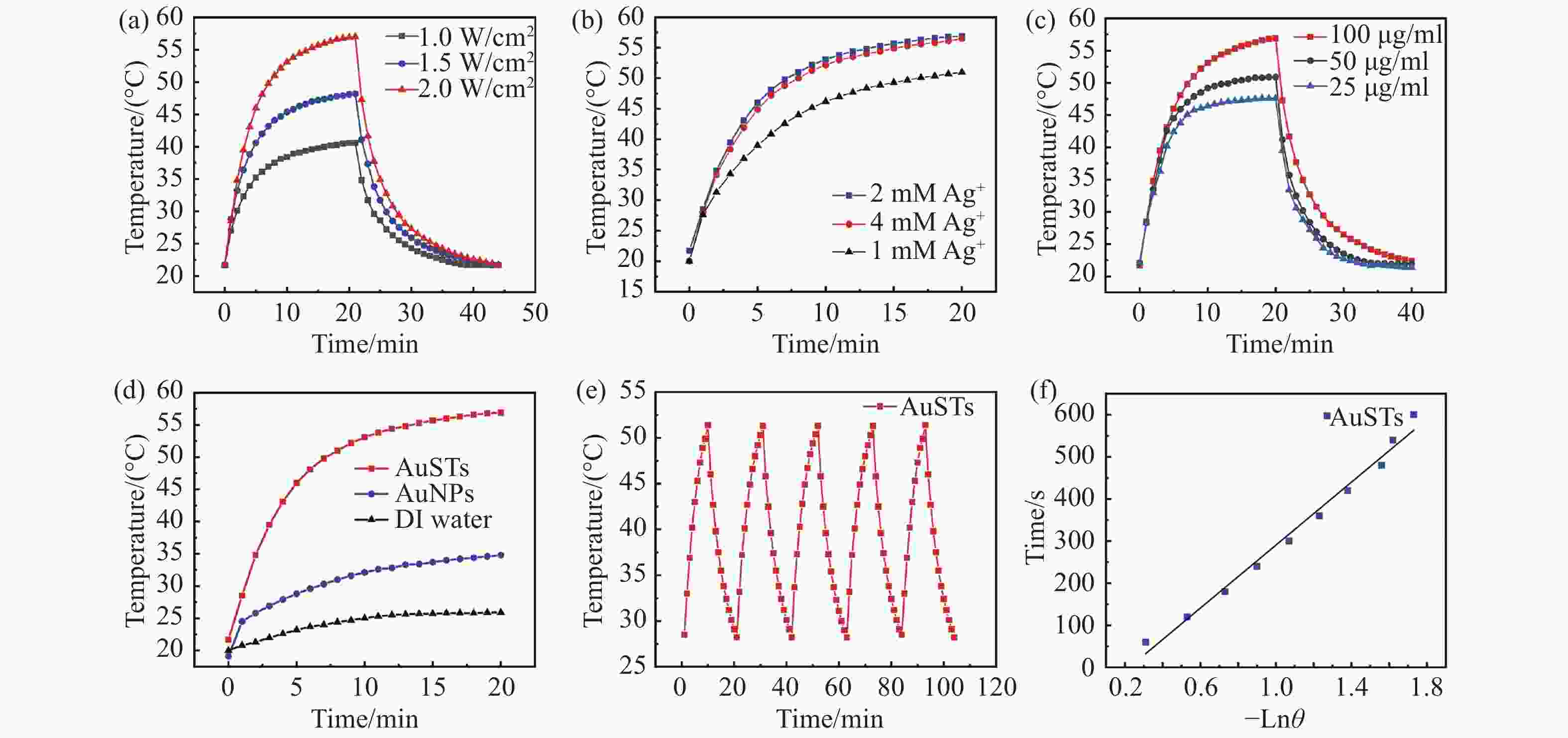
图 2 金纳米星的光热性能测试。(a)AuSTs在808 nm激光不同功率密度下的温度-时间曲线。在808 nm,2 W/cm2激光照射下。(b)不同Ag+浓度制备的AuSTs的温度-时间曲线。(c)不同浓度金纳米星的温度-时间曲线。(d)AuSTs、AuNPs和去离子水的温度-时间曲线。(e)金纳米星系统的光热稳定性测试。(f)金纳米星系统的特征热时间常数
Figure 2. Photothermal property of AuSTs. (a) Temperature-time curves of AuSTs at different power densities. Under 808 nm, 2 W/cm2 laser irradiation. (b) Time-temperature curve of AuSTs prepared with different Ag+ concentrations. (c) Temperature-time curves of AuSTs at different concentrations. (d) Temperature-time curves of AuSTs, AuNPs and DI Water. (e) Photothermal stability of AuSTs. (f) Graph of cooling period of the time versus negative natural logarithm of the temperature
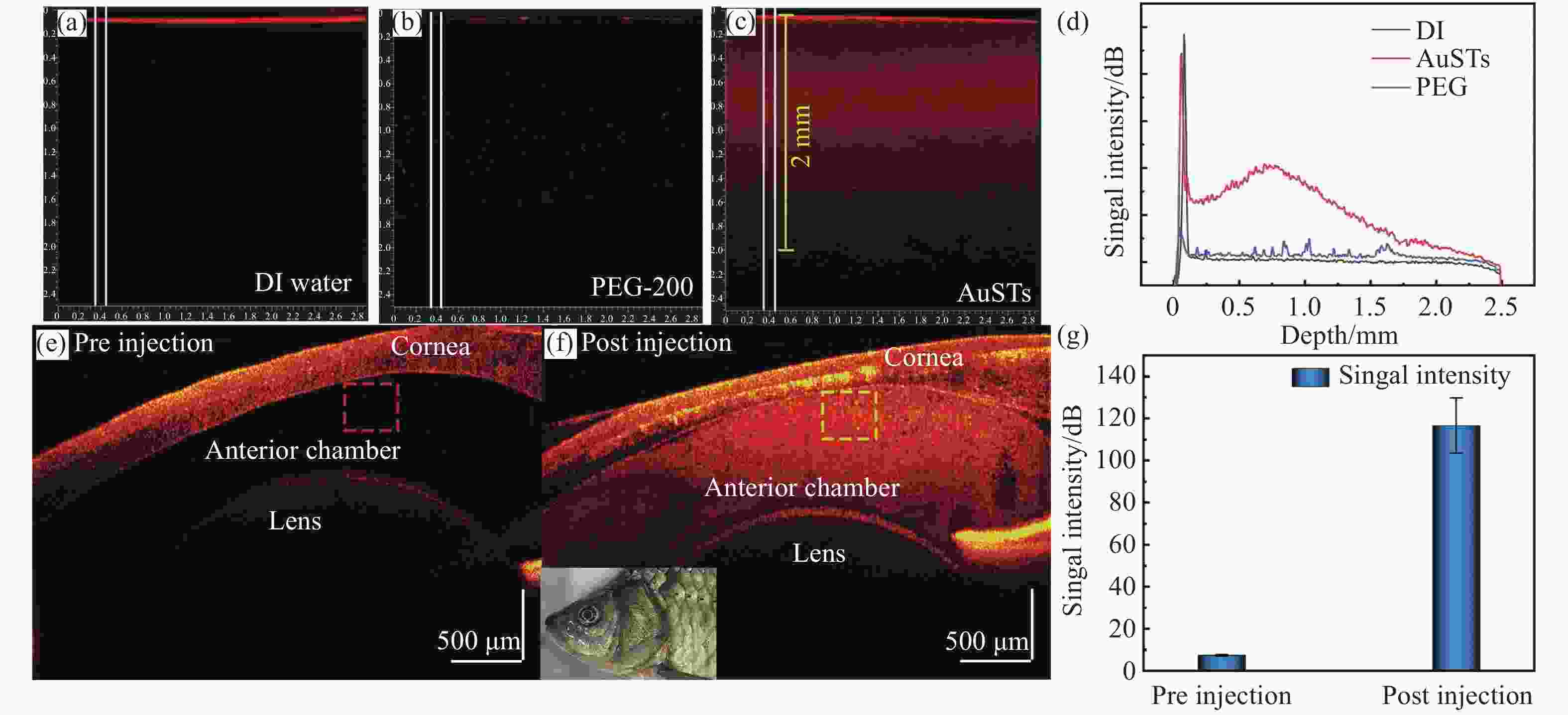
图 5 AuSTs作为造影剂在OCT成像中的效果。(a)加入去离子水的组织体模。(b)嵌入PEG-200的体模成像效果。(c)嵌入AuSTs的体模成像效果。(d)对(a)、(b)和(c)中框选区域的信号强度统计。鲫鱼眼部AuSTs造影剂注射前(e)和注射后(f)的OCT成像效果。(g)对(e)和(f)中框选区域的信号强度统计
Figure 5. OCT imaging with AuSTs as contrast agents. OCT image of simulated tissue with (a) DI water, (b) PEG-200, and (c) AuSTs. (d) OCT signal intensity on depth of simulated tissue with DI water, PEG-200 and AuSTs. The OCT imaging effects of AuSTs in the eyes of crucian carp before (e) and after (f) injection. (g) Statistics on the signal strength of the area selected in (e) and (f)
-
[1] KELKAR S S, REINEKE T M. Theranostics: combining imaging and therapy[J]. Bioconjugate Chemistry, 2011, 22(10): 1879-1903. doi: 10.1021/bc200151q [2] 周莹, 刘赛男, 蔡砺寒, 等. 铁掺杂的聚2-硝基-1, 4-苯二胺纳米球的制备及在光热/光动力/化学动力学肿瘤治疗中的应用[J]. 应用化学,2021,38(2):181-187.ZHOU Y, LIU S N, CAI L H, et al. Ion-doped poly(2-nitro-1, 4-phenylenediamine) nanospheres for synergistic photo- and chemo-dynamic therapy[J]. Chinese Journal of Applied Chemistry, 2021, 38(2): 181-187. (in Chinese) [3] KUMAR A, KIM S, NAM J M. Plasmonically engineered nanoprobes for biomedical applications[J]. Journal of the American Chemical Society, 2016, 138(44): 14509-14525. doi: 10.1021/jacs.6b09451 [4] ZOU Q L, ABBAS M, ZHAO L Y, et al. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy[J]. Journal of the American Chemical Society, 2017, 139(5): 1921-1927. doi: 10.1021/jacs.6b11382 [5] JIANG Y T, SUN M X, OUYANG N, et al. Synergistic chemo-thermal therapy of cancer by DNA-templated silver nanoclusters and polydopamine nanoparticles[J]. ACS Applied Materials &Interfaces, 2021, 13(18): 21653-21660. [6] RAEESI V, CHOU L Y T, CHAN W C W. Tuning the drug loading and release of DNA-assembled gold-nanorod superstructures[J]. Advanced Materials, 2016, 28(38): 8511-8518. doi: 10.1002/adma.201600773 [7] 张雅静, 王晓辉, 徐亚娟, 等. 石墨烯纳米载药体系的制备及对肿瘤细胞的杀伤作用[J]. 应用化学,2021,38(6):693-703.ZHANG Y J, WANG X H, XU Y J, et al. Preparation of graphene Nano-drug carrier system and its killing effect on tumor cells[J]. Chinese Journal of Applied Chemistry, 2021, 38(6): 693-703. (in Chinese) [8] TIAN Q W, TANG M H, SUN Y G, et al. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells[J]. Advanced Materials, 2011, 23(31): 3542-3547. doi: 10.1002/adma.201101295 [9] CHOU S S, KAEHR B, KIM J, et al. Chemically exfoliated MoS2 as near-infrared photothermal agents[J]. Angewandte Chemie International Edition, 2013, 52(15): 4160-4164. doi: 10.1002/anie.201209229 [10] 张萌, 陈东圳, 任研伟, 等. 纳米岛状银膜@金纳米针尖表面增强拉曼散射传感界面及多巴胺分子的传感分析[J]. 应用化学,2021,38(7):866-873.ZHANG M, CHEN D ZH, REN Y W, et al. Sensing interface based on nanoislandlike sliver film@gold nanotip for surface enhanced Raman scattering analysis of dopamine[J]. Chinese Journal of Applied Chemistry, 2021, 38(7): 866-873. (in Chinese) [11] DE ABERASTURI D J, SERRANO-MONTES A B, LIZ-MARZÁN L M. Modern applications of plasmonic nanoparticles: from energy to health[J]. Advanced Optical Materials, 2015, 3(5): 602-617. doi: 10.1002/adom.201500053 [12] PANG B, ZHAO Y F, LUEHMANN H, et al. 64Cu-doped PdCu@Au tripods: a multifunctional nanomaterial for positron emission tomography and image-guided photothermal cancer treatment[J]. ACS Nano, 2016, 10(3): 3121-3131. doi: 10.1021/acsnano.5b07968 [13] RAYALU S S, JOSE D, MANGRULKAR P A, et al. Photodeposition of AuNPs on metal oxides: study of SPR effect and photocatalytic activity[J]. International Journal of Hydrogen Energy, 2014, 39(8): 3617-3624. doi: 10.1016/j.ijhydene.2013.11.120 [14] MA K, LI Y W, WANG ZH G, et al. Core-shell gold nanorod@layered double hydroxide nanomaterial with highly efficient photothermal conversion and its application in antibacterial and tumor therapy[J]. ACS Applied Materials &Interfaces, 2019, 11(33): 29630-29640. [15] DIAGARADJANE P, SHETTY A, WANG J C, et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy[J]. Nano Letter, 2008, 8(5): 1492-1500. doi: 10.1021/nl080496z [16] CHOI W I, KIM J Y, KANG C, et al. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers[J]. ACS Nano, 2011, 5(3): 1995-2003. doi: 10.1021/nn103047r [17] LU W, XIONG CH Y, ZHANG G D, et al. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres[J]. Clinical Cancer Research, 2009, 15(3): 876-886. doi: 10.1158/1078-0432.CCR-08-1480 [18] KIM S H, KIM J H, KANG S W. Nondestructive defect inspection for LCDs using optical coherence tomography[J]. Displays, 2011, 32(5): 325-329. doi: 10.1016/j.displa.2011.04.002 [19] 陆冬筱, 房文汇, 李玉瑶, 等. 光学相干层析成像技术原理及研究进展[J]. 中国光学,2020,13(5):919-935. doi: 10.37188/CO.2020-0037LU D X, FANG W H, LI Y Y, et al. Optical coherence tomography: principles and recent developments[J]. Chinese Optics, 2020, 13(5): 919-935. (in Chinese) doi: 10.37188/CO.2020-0037 [20] EHLERS J P, GUPTA P K, FARSIU S, et al. Evaluation of contrast agents for enhanced visualization in optical coherence tomography[J]. Investigative Ophthalmology &Visual Science, 2010, 51(12): 6614-6619. [21] CHEN ZH P, MILNER T E, SRINIVAS S, et al. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography[J]. Optics Letters, 1997, 22(14): 1119-1121. doi: 10.1364/OL.22.001119 [22] AU K M, LU Z H, MATCHER S J, et al. Polypyrrole nanoparticles: a potential optical coherence tomography contrast agent for cancer imaging[J]. Advanced Materials, 2011, 23(48): 5792-5795. doi: 10.1002/adma.201103190 [23] MONDAL I, RAJ S, ROY P, et al. Silver nanoparticles (AgNPs) as a contrast agent for imaging of animal tissue using swept-source optical coherence tomography (SSOCT)[J]. Laser Physics, 2018, 28(1): 015601. doi: 10.1088/1555-6611/aa884b [24] 邢雅艳, 史宇哲, 邓世贤, 等. 儿茶素-银纳米复合材料的制备及其应用[J]. 应用化学,2020,37(9):1062-1068. doi: 10.11944/j.issn.1000-0518.2020.09.200076XING Y Y, SHI Y ZH, DENG SH X, et al. Preparation and application of catechin-silver nanocomposites[J]. Chinese Journal of Applied Chemistry, 2020, 37(9): 1062-1068. (in Chinese) doi: 10.11944/j.issn.1000-0518.2020.09.200076 [25] ZHU H SH, LIU CH H, LIU X X, et al. A multi-colorimetric immunosensor for visual detection of ochratoxin A by mimetic enzyme etching of gold nanobipyramids[J]. Microchimica Acta, 2021, 188(3): 62. doi: 10.1007/s00604-020-04699-5 [26] JENA B K, RAJ C R. Shape-controlled synthesis of gold nanoprism and nanoperiwinkles with pronounced electrocatalytic activity[J]. The Journal of Physical Chemistry C, 2007, 111(42): 15146-15153. doi: 10.1021/jp072363s [27] YAU O, HÉTU M F, HERR J E, et al. Development of a carotid vulnerable plaque phantom model evaluated by pixel distribution analysis[J]. Ultrasound in Medicine and Biology, 2018, 44(12): 2768-2779. doi: 10.1016/j.ultrasmedbio.2018.07.005 [28] 曹小卫, 陈帅, 鲍敏, 等. 金纳米星的制备、表面修饰及其在生物医学领域的应用研究[J]. 化学进展,2018,30(9):1380-1391.CAO X W, CHEN SH, BAO M, et al. Synthesis and surface modifications of Au nanostars and their applications in biomedical fields[J]. Progress in Chemistry, 2018, 30(9): 1380-1391. (in Chinese) -





 下载:
下载: