Advances in organic fluorescent probes for super-resolution imaging of cellular lipid droplets
-
摘要:
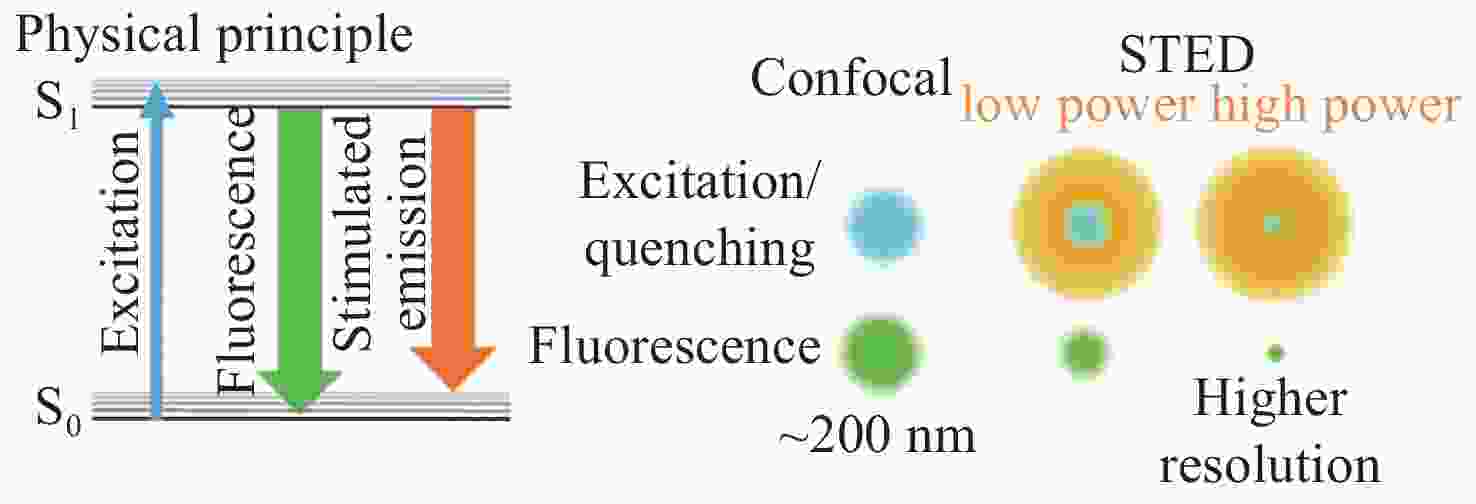
脂滴是真核细胞中必不可少的一种球形细胞器,与很多细胞生理学过程息息相关。荧光成像技术是观察研究脂滴最有力的工具之一。受光学衍射极限的限制,传统的宽场以及共聚焦显微镜所能达到的成像分辨率约为250 nm左右,这对于观测小脂滴,尤其是新生脂滴(尺寸约30~60 nm)来说是远远不够的。在这种情况下,近年来新兴的各种能够打破衍射极限的超分辨荧光显微镜(如受激发射损耗显微镜、结构光照明显微镜以及光激活定位显微镜等)逐渐吸引了科研人员的兴趣。为了得到高分辨率脂滴荧光图像,除了上述超分辨显微镜之外,还需要具有与之相匹配的高性能荧光探针。本文将简要介绍这几种超分辨显微镜的工作原理,讨论其对荧光探针光物理性质的特殊要求,并进一步系统总结脂滴超分辨成像荧光探针的研究进展。与此同时,本文将分析对比不同超分辨显微镜在脂滴荧光成像方面的优势与不足,并对其发展趋势进行展望。
Abstract:Lipid droplets are a kind of spherical organelle in eukaryotic cells and are relevant to many cellular physiological processes. Fluorescence imaging techniques are one of the most powerful tools to visualize and study lipid droplets. However, conventional wide-field microscopy and confocal microscopy can only provide a resolution of about 250 nm due to the limitation of optical diffraction. This resolution is quite insufficient for visualizing the small lipid droplets, especially the nascent ones (size of about 30~60 nm). Emerging super-resolution microscopes that can break the diffraction limit (such as stimulated emission depletion microscopy, structured illumination microscopy and photoactivated localization microscopy) have gradually attracted much interest in recent years. To obtain high-resolution fluorescence images of lipid droplets, the advanced fluorescent probes which meet the special requirements of the corresponding super-resolution microscopes are highly essential. This review paper will briefly introduce the working principles of various super-resolution microscopes, discuss the special requirements on the photophysical properties of fluorescent probes, and systematically summarize the research progress of super-resolution imaging of lipid droplets by employing these fluorescent probes. Meanwhile, this review will compare the advantages and shortcomings of different super-resolution techniques for lipid droplets imaging, and prospect their future possible trends.
-
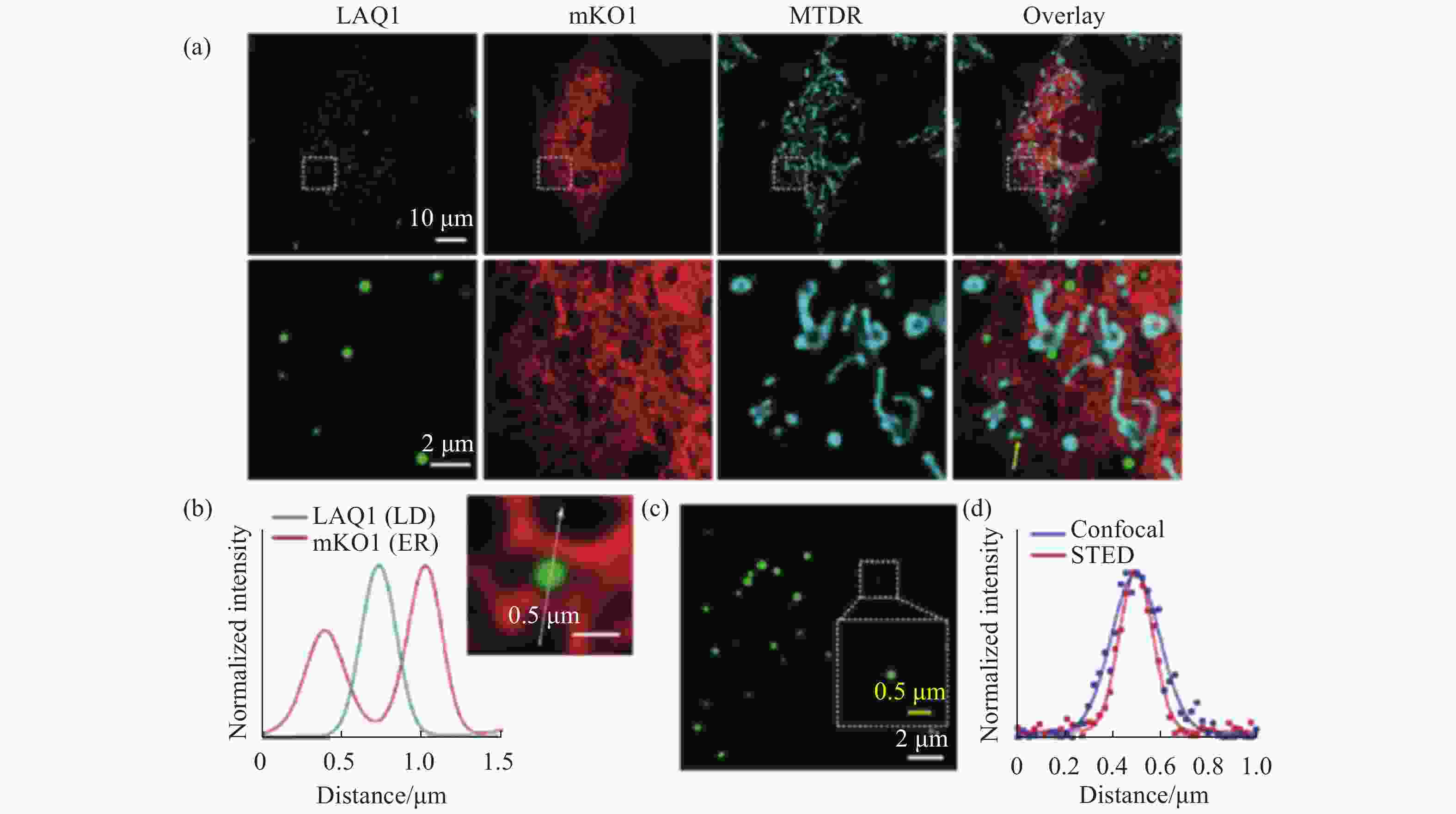
图 3 脂滴荧光探针LAQ1在多色共聚焦成像和STED超分辨成像中的应用。(a)脂滴、内质网和线粒体的三色荧光成像;(b)穿过白色箭头的信号强度分布;(c)小脂滴的STED成像;(d)穿过同一小脂滴的共聚焦和STED成像信号强度图[19]
Figure 3. The applications of lipid droplets fluorescent probe LAQ1 in multicolor confocal imaging and STED super-resolution imaging. (a) Three-color fluorescence imaging of lipid droplets, endoplasmic reticulum and mitochondria; (b) signal intensity profiles across the white arrow; (c) STED imaging of lipid droplets; (d) confocal and STED imaging signal intensity profiles across the same lipid droplet[19]
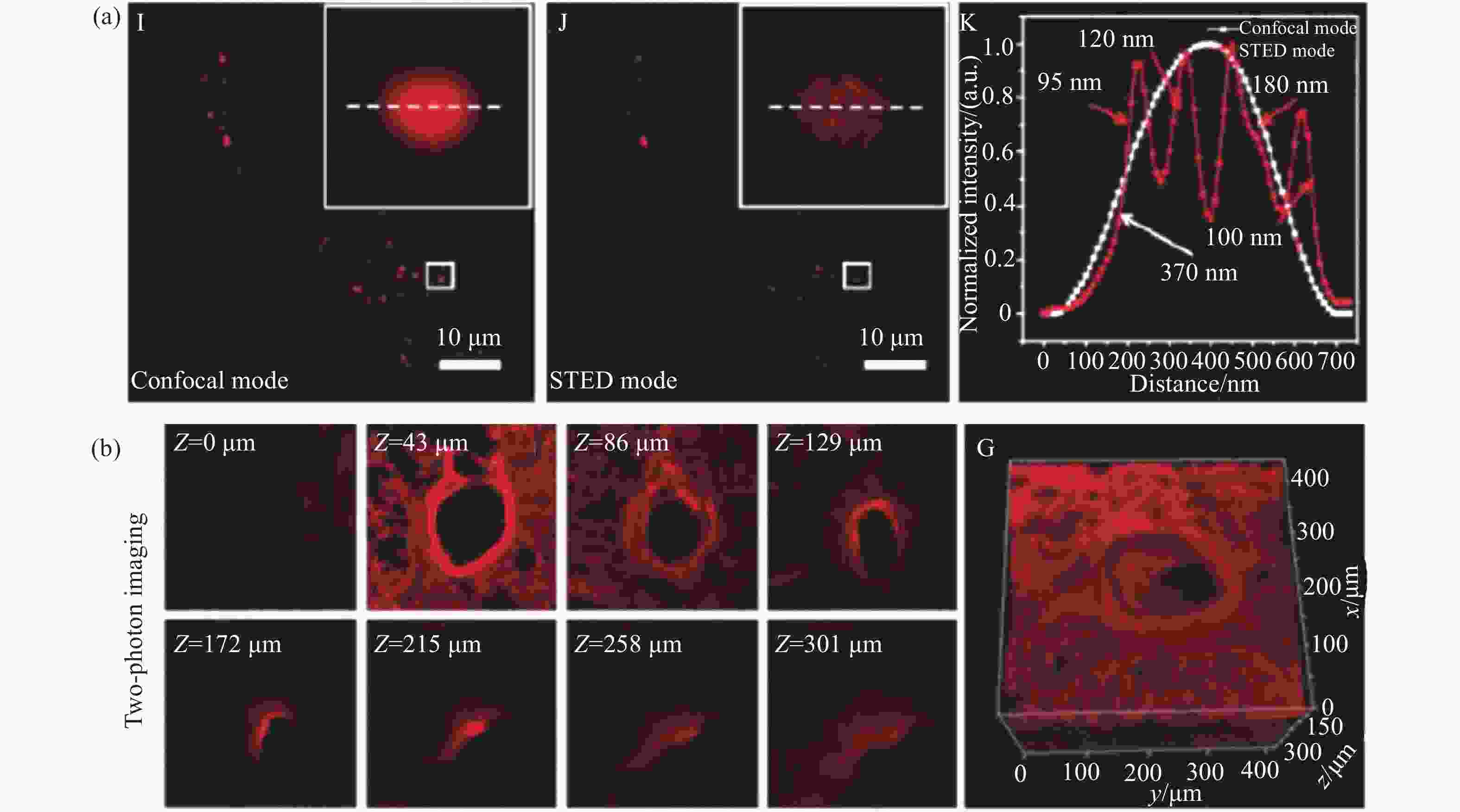
图 5 脂滴荧光探针Lipi-DSB的STED超分辨成像。(a)损耗激光功率依赖的分辨率;(b)与线粒体荧光探针四甲基罗丹明搭配的双色STED成像;(c)连续1000帧的STED超分辨成像动态跟踪;(d)STED超分辨成像动态跟踪纳米尺度新生脂滴的融合过程[21]
Figure 5. STED super-resolution imaging of lipid droplets fluorescent probe Lipi-DSB. (a) STED laser power-dependent resolution; (b) two-color STED imaging of Lipi-DSB paired with mitochondria fluorescent probe tetramethylrhodamine; (c) time-lapse STED super-resolution imaging (1000 consecutive frames); (d) dynamic tracking of nascent lipid droplet fusion processes at the nanoscale by STED super-resolution imaging[21]
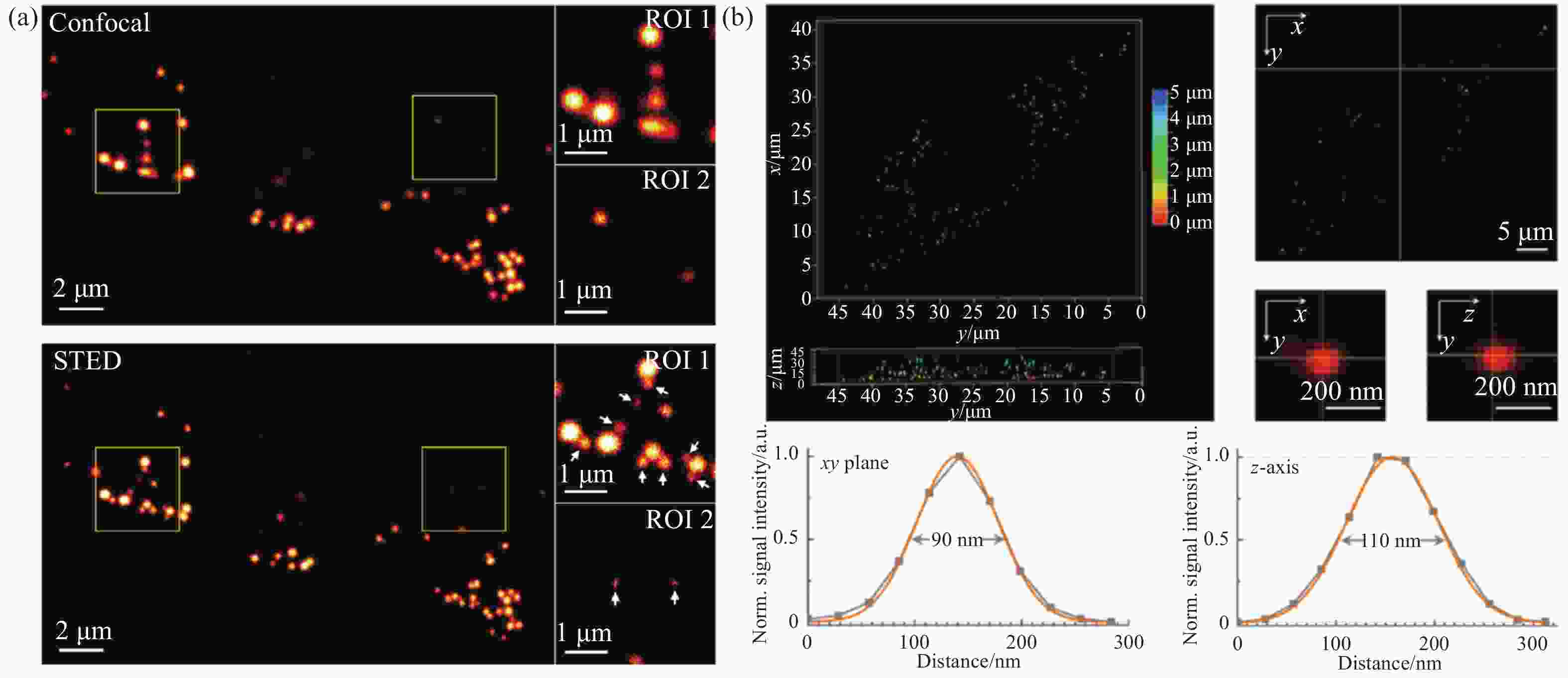
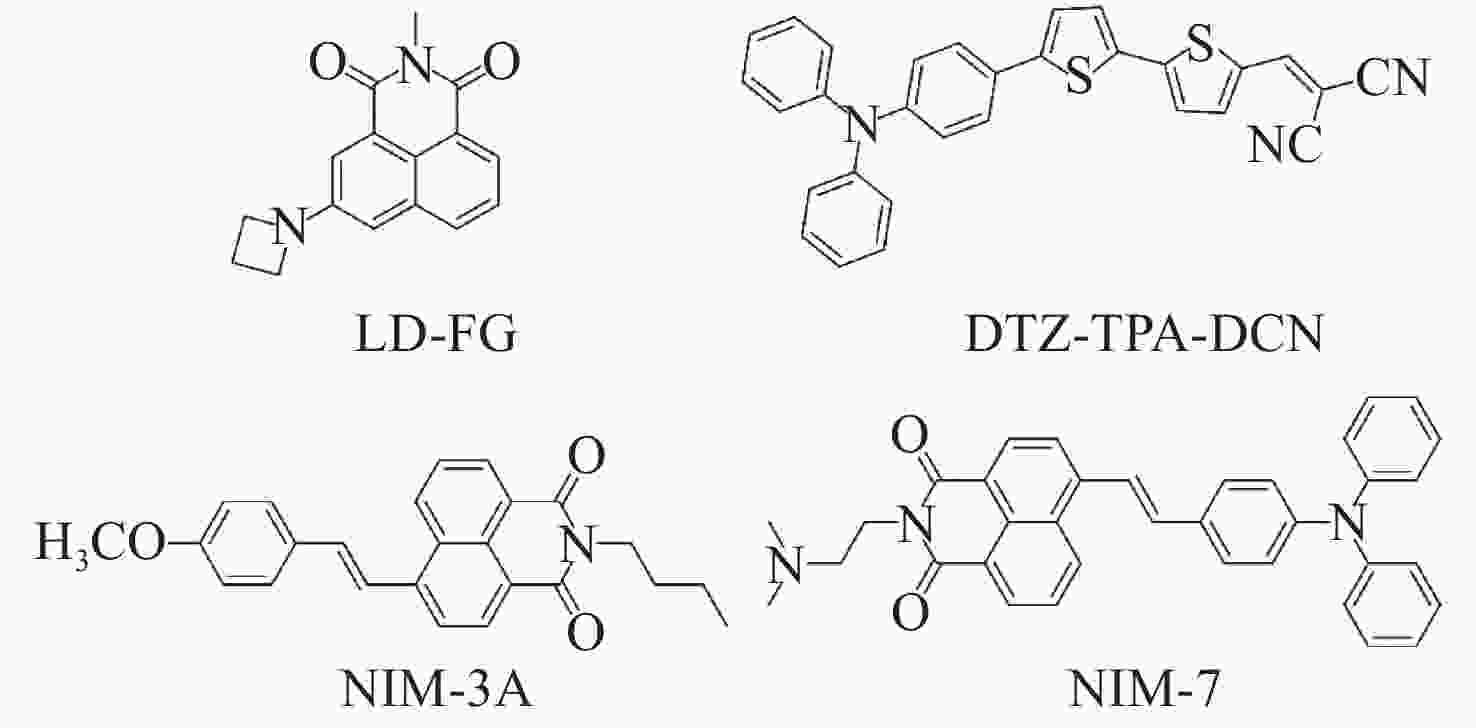
图 9 脂滴荧光探针LD-FG的SIM超分辨成像。(a)缓冲策略提升光稳定性;(b)荧光探针的光开关特性;(c)相邻脂滴间的融合;(d)不相邻脂滴间的融合[27]
Figure 9. SIM super-resolution imaging of the lipid droplets fluorescent probe LD-FG. (a) Buffer strategy enables stable imaging of lipid droplets; (b) fluorescence switching property of the fluorescent probe; (c) coalescence between adjacent lipid droplets; (d) coalescence between non-adjacent lipid droplets[27]
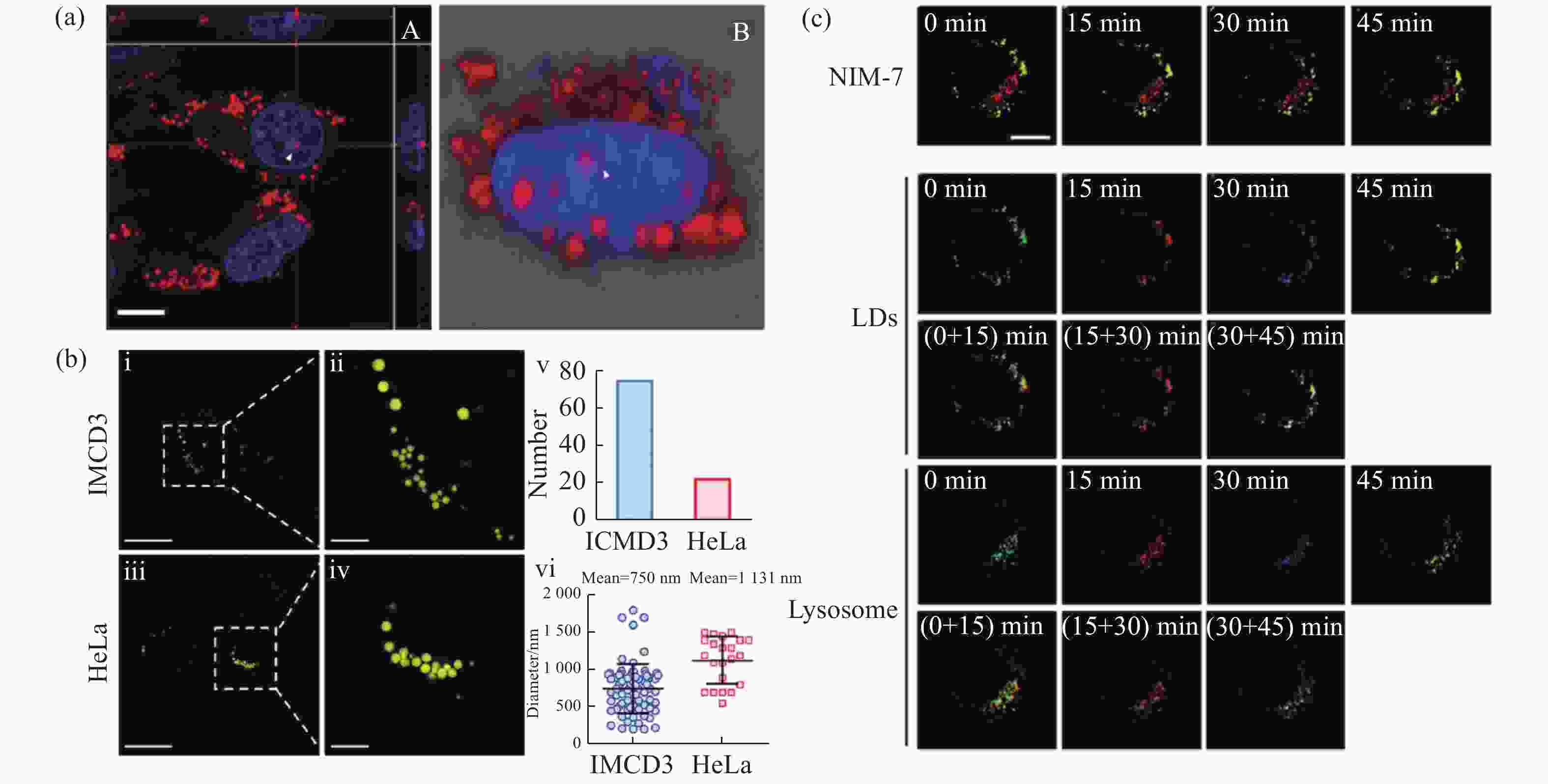
图 10 有机荧光探针在脂滴SIM超分辨成像中的应用。(a)荧光探针DTZ-TPA-DCN用于染色核脂滴[28];(b)荧光探针NIM-3A用于分析细胞内脂滴数量和尺寸[29];(c)荧光探针NIM-7用于追踪脂滴和溶酶体的动态过程[30]
Figure 10. Applications of organic fluorescent probes in SIM super-resolution imaging of lipid droplets. (a) Fluorescent probe DTZ-TPA-DCN used for staining nuclear lipid droplets[28]; (b) fluorescent probe NIM-3A used for analyzing the number and size of lipid droplets[29]; (c) fluorescent probe NIM-7 used for tracking dynamic processes of lipid droplets and lysosomes[30]
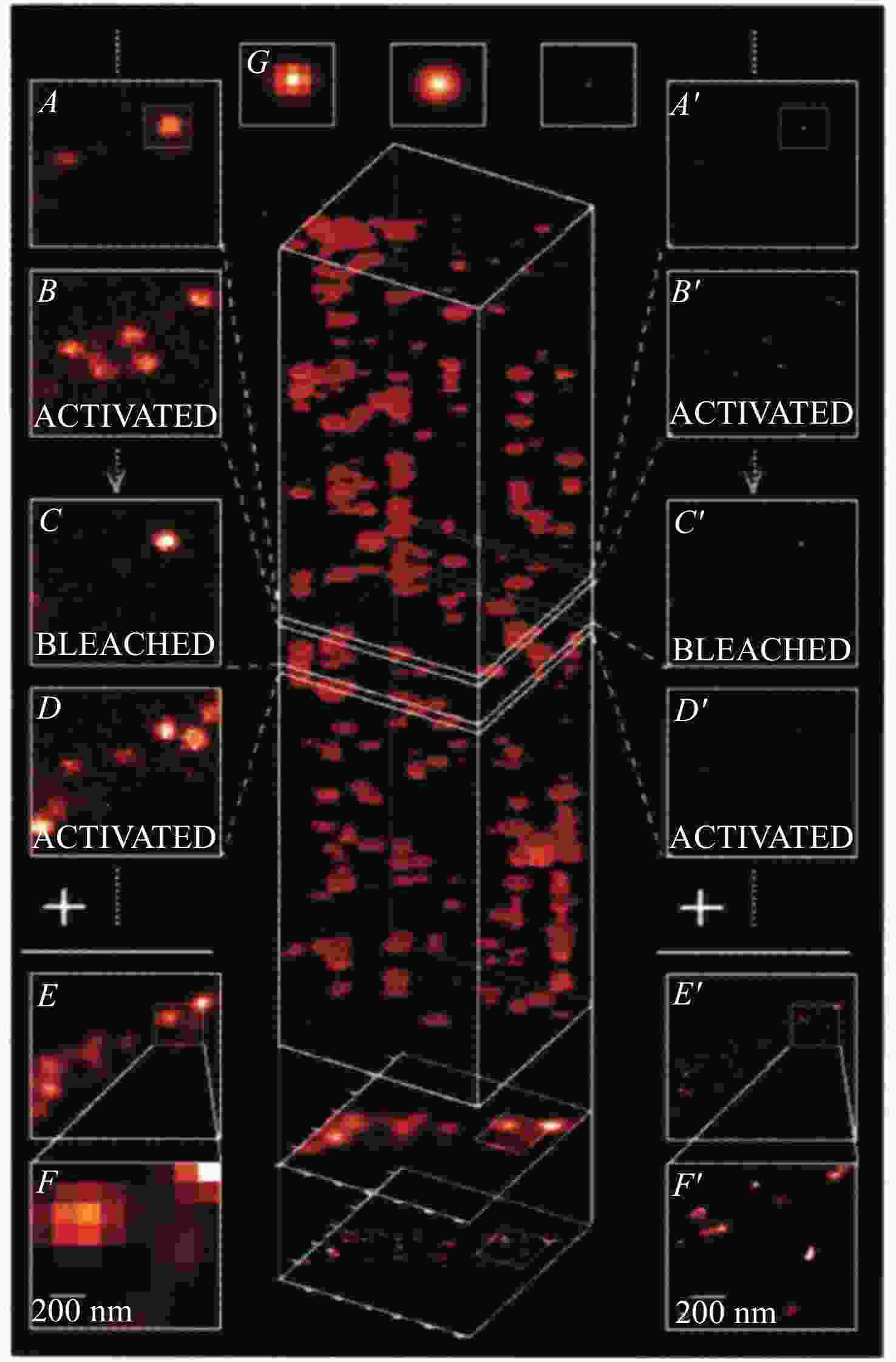
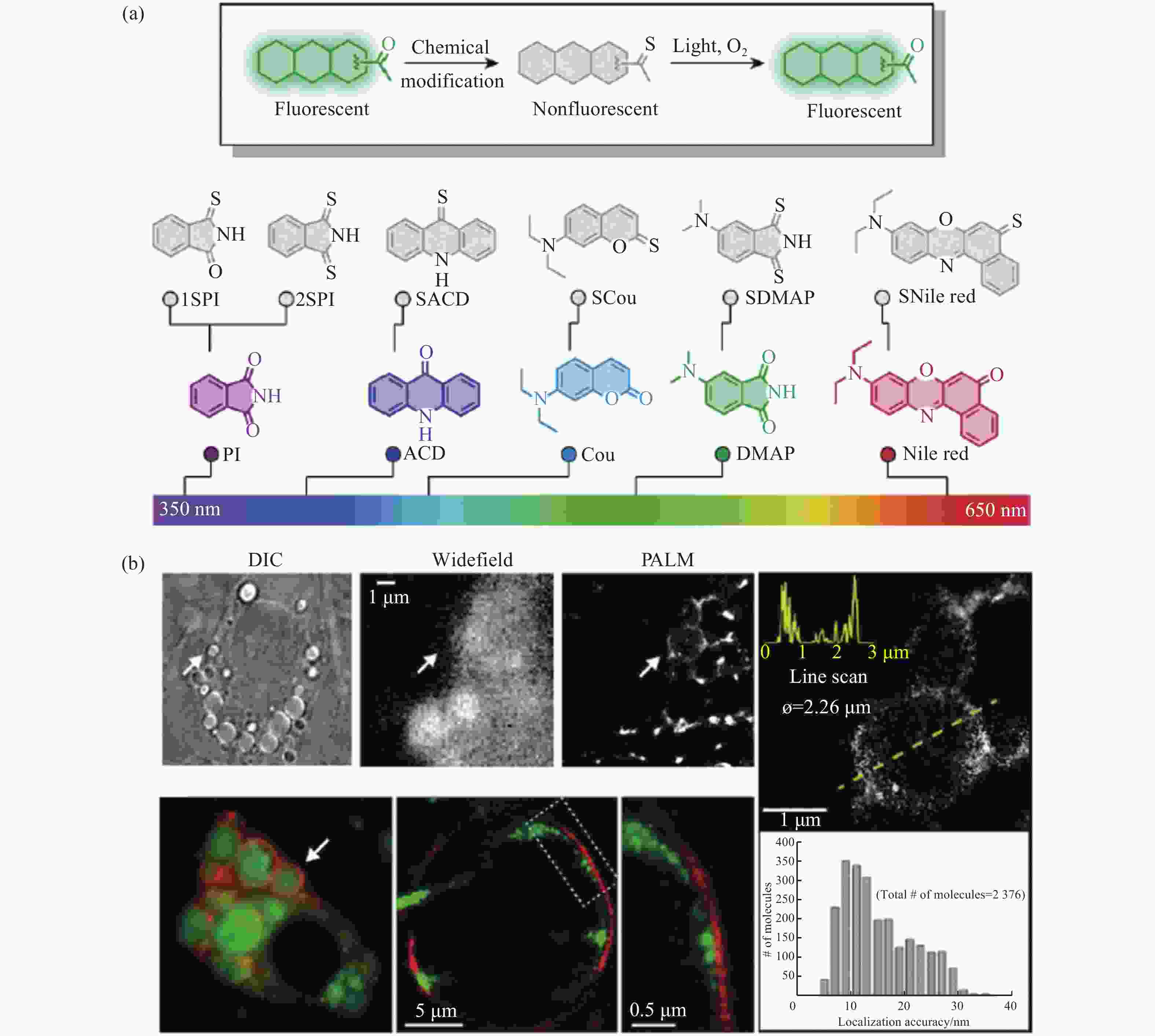
图 12 光开关荧光探针在脂滴PALM超分辨成像中的应用。(a)具有荧光开关特性的有机荧光探针;(b)脂肪细胞中脂滴在宽场和PALM显微镜下的明场和双色成像[31]
Figure 12. Applications of light-switchable fluorescent probes in PALM super-resolution imaging of lipid droplets. (a) The organic fluorescent probes with fluorescence ON/OFF characteristics; (b) bright field and two-color imaging of lipid droplets in adipocyte under wide-field and PALM microscopes[31]
表 1 文献中报道的细胞脂滴超分辨成像结果总结
Table 1. Summary of super-resolution imaging of cell lipid droplets reported in the literatures
发表年份 超分辨成像技术 荧光探针 成像分辨率/nm 是否动态超分辨成像 参考文献 2021 STED LAQ1 166 未提及 19 2021 STED DTPA-BT-M 95 未提及 20 2021 STED Lipi-DSB 58 是 21 2021 STED Lipi-BDTO 65 是 22 2021 SIM LD-FG 180 是 27 2021 SIM DTZ-TPA-DCN 未提及 未提及 28 2018 SIM NIM-3A 未提及 未提及 29 2019 SIM NIM-7 未提及 未提及 30 2019 PALM SNile Red <250 未提及 31 2021 PALM BAD-Oxm 未提及 未提及 32 2020 PALM BODIPY-C12 125 未提及 33 -
[1] OLZMANN J A, CARVALHO P. Dynamics and functions of lipid droplets[J]. Nature Reviews Molecular Cell Biology, 2019, 20(3): 137-155. doi: 10.1038/s41580-018-0085-z [2] THIAM A R, BELLER M. The why, when and how of lipid droplet diversity[J]. Journal of Cell Science, 2017, 130(2): 315-324. [3] CHOUDHARY V, OJHA N, GOLDEN A, et al. A conserved family of proteins facilitates nascent lipid droplet budding from the ER[J]. Journal of Cell Biology, 2015, 211(2): 261-271. doi: 10.1083/jcb.201505067 [4] JACQUIER N, CHOUDHARY V, MARI M, et al. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae[J]. Journal of Cell Science, 2011, 124(14): 2424-2437. doi: 10.1242/jcs.076836 [5] KASSAN A, HERMS A, FERNÁNDEZ-VIDAL A, et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains[J]. Journal of Cell Biology, 2013, 203(6): 985-1001. doi: 10.1083/jcb.201305142 [6] BUHMAN K K, CHEN H C, FARESE R V JR. The enzymes of neutral lipid synthesis[J]. Journal of Biological Chemistry, 2001, 276(44): 40369-40372. doi: 10.1074/jbc.R100050200 [7] THIAM A R, FARESE R V JR, WALTHER T C. The biophysics and cell biology of lipid droplets[J]. Nature Reviews Molecular Cell Biology, 2013, 14(12): 775-786. doi: 10.1038/nrm3699 [8] WALTHER T C, FARESE R V JR. Lipid droplets and cellular lipid metabolism[J]. Annual Review of Biochemistry, 2012, 81: 687-714. doi: 10.1146/annurev-biochem-061009-102430 [9] FARESE R V JR, WALTHER T C. Lipid droplets finally get a little R-E-S-P-E-C-T[J]. Cell, 2009, 139(5): 855-860. doi: 10.1016/j.cell.2009.11.005 [10] ROITENBERG N, COHEN E. Lipid assemblies at the crossroads of aging, proteostasis, and neurodegeneration[J]. Trends in Cell Biology, 2019, 29(12): 954-963. doi: 10.1016/j.tcb.2019.09.003 [11] KRAHMER N, FARESE R V JR, WALTHER T C. Balancing the fat: lipid droplets and human disease[J]. EMBO Molecular Medicine, 2013, 5(7): 973-983. doi: 10.1002/emmm.201100671 [12] ONAL G, KUTLU O, GOZUACIK D, et al. Lipid droplets in health and disease[J]. Lipids in Health and Disease, 2017, 16(1): 128. doi: 10.1186/s12944-017-0521-7 [13] LIU Q P, LUO Q, HALIM A, et al. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer[J]. Cancer Letters, 2017, 401: 39-45. doi: 10.1016/j.canlet.2017.05.002 [14] COLLOT M, FAM T K, ASHOKKUMAR P, et al. Ultrabright and fluorogenic probes for multicolor imaging and tracking of lipid droplets in cells and tissues[J]. Journal of the American Chemical Society, 2018, 140(16): 5401-5411. doi: 10.1021/jacs.7b12817 [15] GUO L F, TIAN M G, ZHANG ZH Y, et al. Simultaneous two-color visualization of lipid droplets and endoplasmic reticulum and their interplay by single fluorescent probes in lambda mode[J]. Journal of the American Chemical Society, 2021, 143(8): 3169-3179. doi: 10.1021/jacs.0c12323 [16] SHI L, LI K, LI L L, et al. Novel easily available purine-based AIEgens with colour tunability and applications in lipid droplet imaging[J]. Chemical Science, 2018, 9(48): 8969-8974. doi: 10.1039/C8SC03369B [17] ZHANG CH, LI J J, LAN L, et al. Quantification of lipid metabolism in living cells through the dynamics of lipid droplets measured by stimulated raman scattering imaging[J]. Analytical Chemistry, 2017, 89(8): 4502-4507. doi: 10.1021/acs.analchem.6b04699 [18] ZHANG CH, BOPPART S A. Dynamic signatures of lipid droplets as new markers to quantify cellular metabolic changes[J]. Analytical Chemistry, 2020, 92(24): 15943-15952. doi: 10.1021/acs.analchem.0c03366 [19] TAKI M, KAJIWARA K, YAMAGUCHI E, et al. Fused thiophene-S, S-dioxide-based super-photostable fluorescent marker for lipid droplets[J]. ACS Materials Letters, 2021, 3(1): 42-49. doi: 10.1021/acsmaterialslett.0c00451 [20] XU Y Z, ZHANG H K, ZHANG N, et al. An easily synthesized AIE luminogen for lipid droplet-specific super-resolution imaging and two-photon imaging[J]. Materials Chemistry Frontiers, 2021, 5(4): 1872-1883. doi: 10.1039/D0QM00682C [21] ZHOU R, WANG CH G, LIANG X SH, et al. Stimulated emission depletion (STED) super-resolution imaging with an advanced organic fluorescent probe: visualizing the cellular lipid droplets at the unprecedented nanoscale resolution[J]. ACS Materials Letters, 2021, 3(5): 516-524. doi: 10.1021/acsmaterialslett.1c00143 [22] LIU G N, PENG G SH, DAI J N, et al. STED nanoscopy imaging of cellular lipid droplets employing a superior organic fluorescent probe[J]. Analytical Chemistry, 2021, 93(44): 14784-14791. doi: 10.1021/acs.analchem.1c03474 [23] LIU G N, DAI J N, ZHOU R, et al. A distyrylbenzene-based fluorescent probe with high photostability and large Stokes shift for STED nanoscopy imaging of cellular lipid droplets[J]. Sensors and Actuators B:Chemical, 2022, 353: 131000. doi: 10.1016/j.snb.2021.131000 [24] XU H K, ZHANG H H, LIU G, et al. Coumarin-based fluorescent probes for super-resolution and dynamic tracking of lipid droplets[J]. Analytical Chemistry, 2019, 91(1): 977-982. doi: 10.1021/acs.analchem.8b04079 [25] O’CONNOR D, BYRNE A, BERSELLI G B, et al. Mega-stokes pyrene ceramide conjugates for STED imaging of lipid droplets in live cells[J]. Analyst, 2019, 144(5): 1608-1621. doi: 10.1039/C8AN02260G [26] LIU X L, XIN L, TONG Z, et al. Revealing lipid droplets evolution at nanoscale under proteohormone stimulation by a BODIPY-hexylcarbazole derivative[J]. Biosensors and Bioelectronics, 2021, 175: 112871. doi: 10.1016/j.bios.2020.112871 [27] CHEN J, WANG CH, LIU W J, et al. Stable super-resolution imaging of lipid droplet dynamics through a buffer strategy with a hydrogen-bond sensitive fluorogenic probe[J]. Angewandte Chemie International Edition, 2021, 60(47): 25104-25113. doi: 10.1002/anie.202111052 [28] WU M Y, LEUNG J K, KAM C, et al. A near-infrared AIE probe for super-resolution imaging and nuclear lipid droplet dynamic study[J]. Materials Chemistry Frontiers, 2021, 5(7): 3043-3049. doi: 10.1039/D0QM00914H [29] ZHENG X J, ZHU W CH, NI F, et al. A specific bioprobe for super-resolution fluorescence imaging of lipid droplets[J]. Sensors and Actuators B:Chemical, 2018, 255: 3148-3154. doi: 10.1016/j.snb.2017.09.139 [30] ZHENG X J, ZHU W CH, NI F, et al. Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe[J]. Chemical Science, 2019, 10(8): 2342-2348. doi: 10.1039/C8SC04462G [31] TANG J, ROBICHAUX M A, WU K L, et al. Single-atom fluorescence switch: a general approach toward visible-light-activated dyes for biological imaging[J]. Journal of the American Chemical Society, 2019, 141(37): 14699-14706. doi: 10.1021/jacs.9b06237 [32] WANG L SH, WANG SH CH, TANG J, et al. Oxime as a general photocage for the design of visible light photo-activatable fluorophores[J]. Chemical Science, 2021, 12(47): 15572-15580. doi: 10.1039/D1SC05351E [33] ADHIKARI S, BANERJEE C, MOSCATELLI J, et al. Conventional BODIPY conjugates for live-cell super-resolution microscopy and single-molecule tracking[J]. Journal of Visualized Experiments, 2020(160): 60950-60958. [34] YE SH, YAN W, ZHAO M J, et al. Low-saturation-intensity, high-photostability, and high-resolution STED nanoscopy assisted by CsPbBr3 quantum dots[J]. Advanced Materials, 2018, 30(23): 1800167. doi: 10.1002/adma.201800167 [35] WANG L W, CHEN Y, PENG X, et al. Ultralow power demand in fluorescence nanoscopy with digitally enhanced stimulated emission depletion[J]. Nanophotonics, 2020, 9(4): 831-839. doi: 10.1515/nanoph-2019-0475 [36] LI D Y, QIN W, XU B, et al. AIE nanoparticles with high stimulated emission depletion efficiency and photobleaching resistance for long-term super-resolution bioimaging[J]. Advanced Materials, 2017, 29(43): 1703643. doi: 10.1002/adma.201703643 [37] LI D Y, NI X, ZHANG X Y, et al. Aggregation-induced emission luminogen-assisted stimulated emission depletion nanoscopy for super-resolution mitochondrial visualization in live cells[J]. Nano Research, 2018, 11(11): 6023-6033. doi: 10.1007/s12274-018-2118-5 [38] LIU Y J, LU Y Q, YANG X S, et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy[J]. Nature, 2017, 543(7644): 229-233. doi: 10.1038/nature21366 [39] HUANG X SH, FAN J CH, LI L J, et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy[J]. Nature Biotechnology, 2018, 36(5): 451-459. doi: 10.1038/nbt.4115 [40] ZHENG X L, DUAN R Y, LI L J, et al. Live-cell superresolution pathology reveals different molecular mechanisms of Pelizaeus-Merzbacher disease[J]. Science Bulletin, 2020, 65(24): 2061-2064. doi: 10.1016/j.scib.2020.08.016 [41] ZHANGHAO H, CHEN X Y, LI M H, et al. Super-resolution imaging of fluorescent dipoles via polarized structured illumination microscopy[J]. Nature Communications, 2019, 10(1): 4694. doi: 10.1038/s41467-019-12681-w [42] GUO Y T, LI D, ZHANG S W, et al. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales[J]. Cell, 2018, 175(5): 1430-1442.e17. doi: 10.1016/j.cell.2018.09.057 [43] LI D, SHAO L, CHEN B CH, et al. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics[J]. Science, 2015, 349(6251): aab3500. doi: 10.1126/science.aab3500 [44] ZHAO T Y, HAO H W, WANG ZH J, et al. Multi-color structured illumination microscopy for live cell imaging based on the enhanced image recombination transform algorithm[J]. Biomedical Optics Express, 2021, 12(6): 3474-3484. doi: 10.1364/BOE.423171 [45] WANG ZH J, ZHAO T Y, HAO H W, et al. High-speed image reconstruction for optically sectioned, super-resolution structured illumination microscopy[J]. Advanced Photonics, 2022, 4(2): 026003. [46] LIU ZH H, LIU J, WANG X D, et al. Fluorescent bioconjugates for super-resolution optical nanoscopy[J]. Bioconjugate Chemistry, 2020, 31(8): 1857-1872. doi: 10.1021/acs.bioconjchem.0c00320 [47] GUI D, CHEN Y J, KUANG W B, et al. Accelerating multi-emitter localization in super-resolution localization microscopy with FPGA-GPU cooperative computation[J]. Optics Express, 2021, 29(22): 35247-35260. doi: 10.1364/OE.439976 [48] WANG Y J, KUANG W B, SHANG M T, et al. Two-color super-resolution localization microscopy via joint encoding of emitter location and color[J]. Optics Express, 2021, 29(21): 34797-34809. doi: 10.1364/OE.440706 [49] DU Y, WANG CH Z, ZHANG CH, et al. Computational framework for generating large panoramic super-resolution images from localization microscopy[J]. Biomedical Optics Express, 2021, 12(8): 4759-4778. doi: 10.1364/BOE.433489 [50] HELL S W, WICHMANN J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy[J]. Optics Letters, 1994, 19(11): 780-782. doi: 10.1364/OL.19.000780 [51] KLAR T A, HELL S W. Subdiffraction resolution in far-field fluorescence microscopy[J]. Optics Letters, 1999, 24(14): 954-956. doi: 10.1364/OL.24.000954 [52] BUTKEVICH A N, YU G, SIDENSTEIN S C, et al. Fluorescent rhodamines and fluorogenic carbopyronines for super-resolution STED microscopy in living cells[J]. Angewandte Chemie International Edition, 2016, 55(10): 3290-3294. doi: 10.1002/anie.201511018 [53] BORDENAVE M D, BALZAROTTI F, STEFANI F D, et al. STED nanoscopy with wavelengths at the emission maximum[J]. Journal of Physics D:Applied Physics, 2016, 49(36): 365102. doi: 10.1088/0022-3727/49/36/365102 [54] GÖTTFERT F, PLEINER T, HEINE J, et al. Strong signal increase in STED fluorescence microscopy by imaging regions of subdiffraction extent[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(9): 2125-2130. doi: 10.1073/pnas.1621495114 [55] SHANK N I, PHAM H H, WAGGONER A S, et al. Twisted cyanines: a non-planar fluorogenic dye with superior photostability and its use in a protein-based fluoromodule[J]. Journal of the American Chemical Society, 2013, 135(1): 242-251. doi: 10.1021/ja308629w [56] SHANK N I, ZANOTTI K J, LANNI F, et al. Enhanced photostability of genetically encodable fluoromodules based on fluorogenic cyanine dyes and a promiscuous protein partner[J]. Journal of the American Chemical Society, 2009, 131(36): 12960-12969. doi: 10.1021/ja9016864 [57] OYAMA Y, MAMADA M, SHUKLA A, et al. Design strategy for robust organic semiconductor laser dyes[J]. ACS Materials Letters, 2020, 2(2): 161-167. doi: 10.1021/acsmaterialslett.9b00536 [58] MICHIE M S, GÖTZ R, FRANKE C, et al. Cyanine conformational restraint in the far-red range[J]. Journal of the American Chemical Society, 2017, 139(36): 12406-12409. doi: 10.1021/jacs.7b07272 [59] ZHOU R, CUI Y Y, DAI J N, et al. A red-emissive fluorescent probe with a compact single-benzene-based skeleton for cell imaging of lipid droplets[J]. Advanced Optical Materials, 2020, 8(13): 1902123. doi: 10.1002/adom.201902123 [60] YANG X S, YANG ZH G, WU ZH Y, et al. Mitochondrial dynamics quantitatively revealed by STED nanoscopy with an enhanced squaraine variant probe[J]. Nature Communications, 2020, 11(1): 3699. doi: 10.1038/s41467-020-17546-1 [61] LIU Y J, DING Y CH, ALONAS E, et al. Achieving λ/10 resolution CW STED nanoscopy with a Ti: sapphire oscillator[J]. PLoS One, 2012, 7(6): e40003. doi: 10.1371/journal.pone.0040003 [62] BIANCHINI P, HARKE B, GALIANI S, et al. Single-wavelength two-photon excitation-stimulated emission depletion (SW2PE-STED) superresolution imaging[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(17): 6390-6393. doi: 10.1073/pnas.1119129109 [63] GUSTAFSSON M G L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy: short communication[J]. Journal of Microscopy, 2000, 198(2): 82-87. doi: 10.1046/j.1365-2818.2000.00710.x [64] GUSTAFSSON M G L. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(37): 13081-13086. doi: 10.1073/pnas.0406877102 [65] 骆清铭, 张镇西. 生物医学光子学[M]. 北京: 人民卫生出版社, 2018.LUO Q M, ZHANG Z X. Biomedical Photonics[M]. Beijing: People's Medical Publishing House, 2018. (in Chinese) [66] 刘志贺, 吴长锋. 超分辨率成像荧光探针材料应用进展[J]. 中国光学,2018,11(3):344-362. doi: 10.3788/co.20181103.0344LIU ZH H, WU CH F. Advances in application of materials of super-resolution imaging fluorescent probe[J]. Chinese Optics, 2018, 11(3): 344-362. (in Chinese) doi: 10.3788/co.20181103.0344 [67] SPAHN C, GRIMM J B, LAVIS L D, et al. Whole-cell, 3D, and multicolor STED imaging with exchangeable fluorophores[J]. Nano Letters, 2019, 19(1): 500-505. doi: 10.1021/acs.nanolett.8b04385 [68] BETZIG E, PATTERSON G H, SOUGRAT R, et al. Imaging intracellular fluorescent proteins at nanometer resolution[J]. Science, 2006, 313(5793): 1642-1645. doi: 10.1126/science.1127344 [69] BRIEKE C, ROHRBACH F, GOTTSCHALK A, et al. Light-controlled tools[J]. Angewandte Chemie International Edition, 2012, 51(34): 8446-8476. doi: 10.1002/anie.201202134 [70] LI W H, ZHENG G H. Photoactivatable fluorophores and techniques for biological imaging applications[J]. Photochemical &Photobiological Sciences, 2012, 11(3): 460-471. [71] SENGUPTA P, VAN ENGELENBURG S B, LIPPINCOTT-SCHWARTZ J. Superresolution imaging of biological systems using photoactivated localization microscopy[J]. Chemical Reviews, 2014, 114(6): 3189-3202. doi: 10.1021/cr400614m [72] NANI R R, GORKA A P, NAGAYA T, et al. Near-IR light-mediated cleavage of antibody-drug conjugates using cyanine photocages[J]. Angewandte Chemie International Edition, 2015, 54(46): 13635-13638. doi: 10.1002/anie.201507391 [73] GWOSCH K C, PAPE J K, BALZAROTTI F, et al. MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells[J]. Nature Methods, 2020, 17(2): 217-224. doi: 10.1038/s41592-019-0688-0 -





 下载:
下载: