-
摘要:
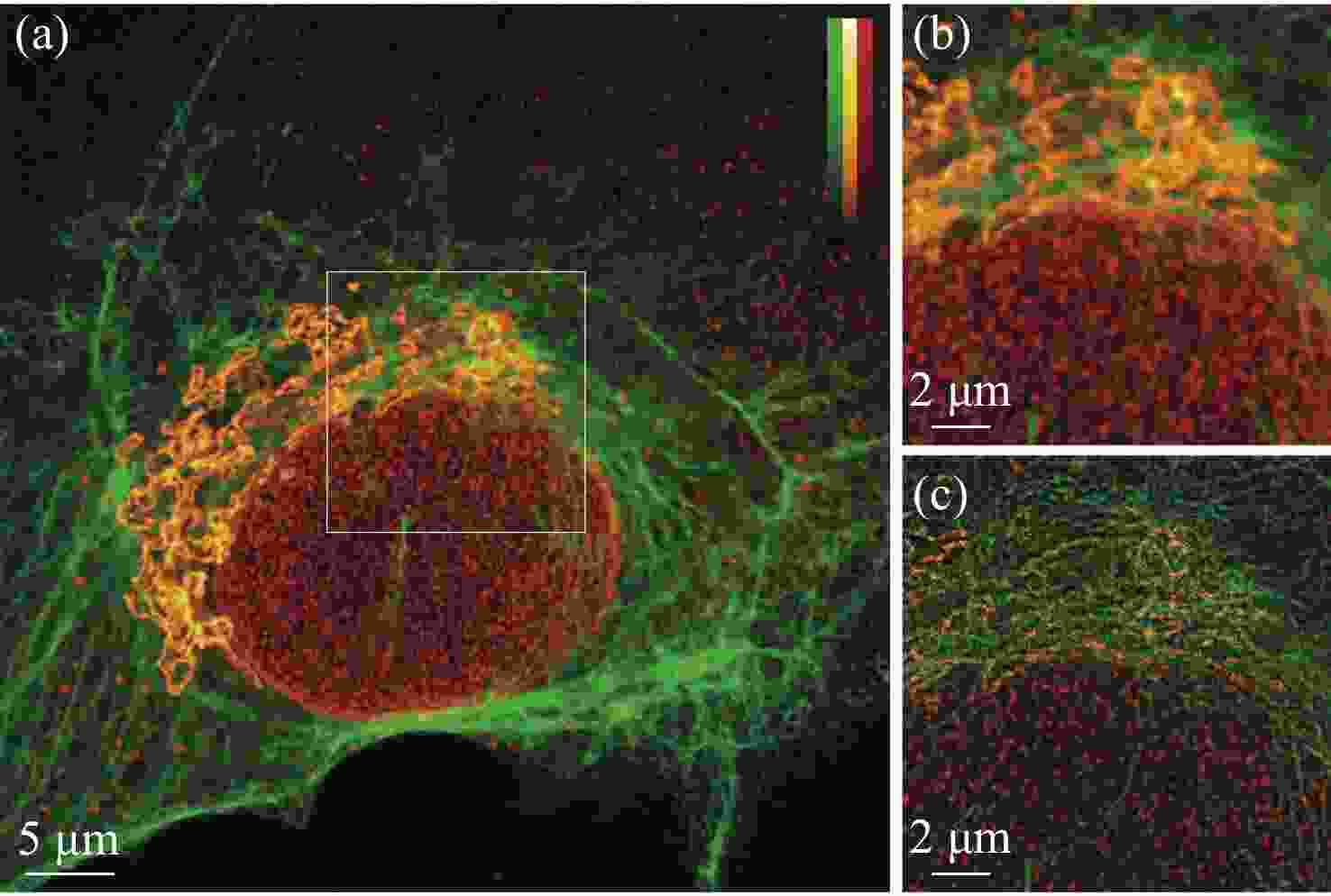
荧光辐射差分显微成像是一种荧光染料普适性强、光毒性较低的超分辨成像技术。然而传统荧光辐射差分成像由于受其成像原理限制,系统复杂度较高、稳定性低且成像速度受限。针对上述问题,本文设计搭建了一套多色虚拟荧光差分显微系统,并对该系统的成像方法和参数间的制约关系进行了分析,基于已有的多色虚拟荧光辐射差分显微术原理,进一步考虑了信噪比和背景噪声等的影响,建立了可通过实验验证的虚拟荧光辐射差分显微成像模型。实验表明,本系统与方法具有结构简单、背景去噪能力强、荧光染料普适性强以及光毒性低等特性,成像分辨率较共聚焦系统提升了1.9倍,成像速度较传统的荧光辐射差分显微系统提升一倍,在3个波长上均获得了良好的成像效果,并在生物细胞成像中得到实验验证。
Abstract:Fluorescence emission difference microscopy is a super-resolution imaging technique with strong universality of fluorescent dyes and low phototoxicity. However, due to the limitation of its principle, traditional fluorescence emission difference microscopy has a high system complexity, low stability and limited imaging speed. In order to improve these defects, we design and build a set of multi-color virtual fluorescence difference microscopy system, and it’s imaging method and parameter are analyzed. On the basis of the existing principle of multi-color virtual fluorescence emission difference microscopy, the influence of the signal-to-noise ratio and background is further considered, and a virtual fluorescence emission difference microscopy imaging model that can be verified experimentally is established. The experiments show that the system and method have the characteristics of simple structure, strong background denoising ability, strong universality of fluorescent dyes, and low phototoxicity. Its imaging resolution is 1.9 times higher than that of confocal system, and its imaging speed is doubled compared to the traditional fluorescence emission difference microscopy system. It has obtained good imaging results at three wavelengths, and has been experimentally verified in biological cell imaging.
-
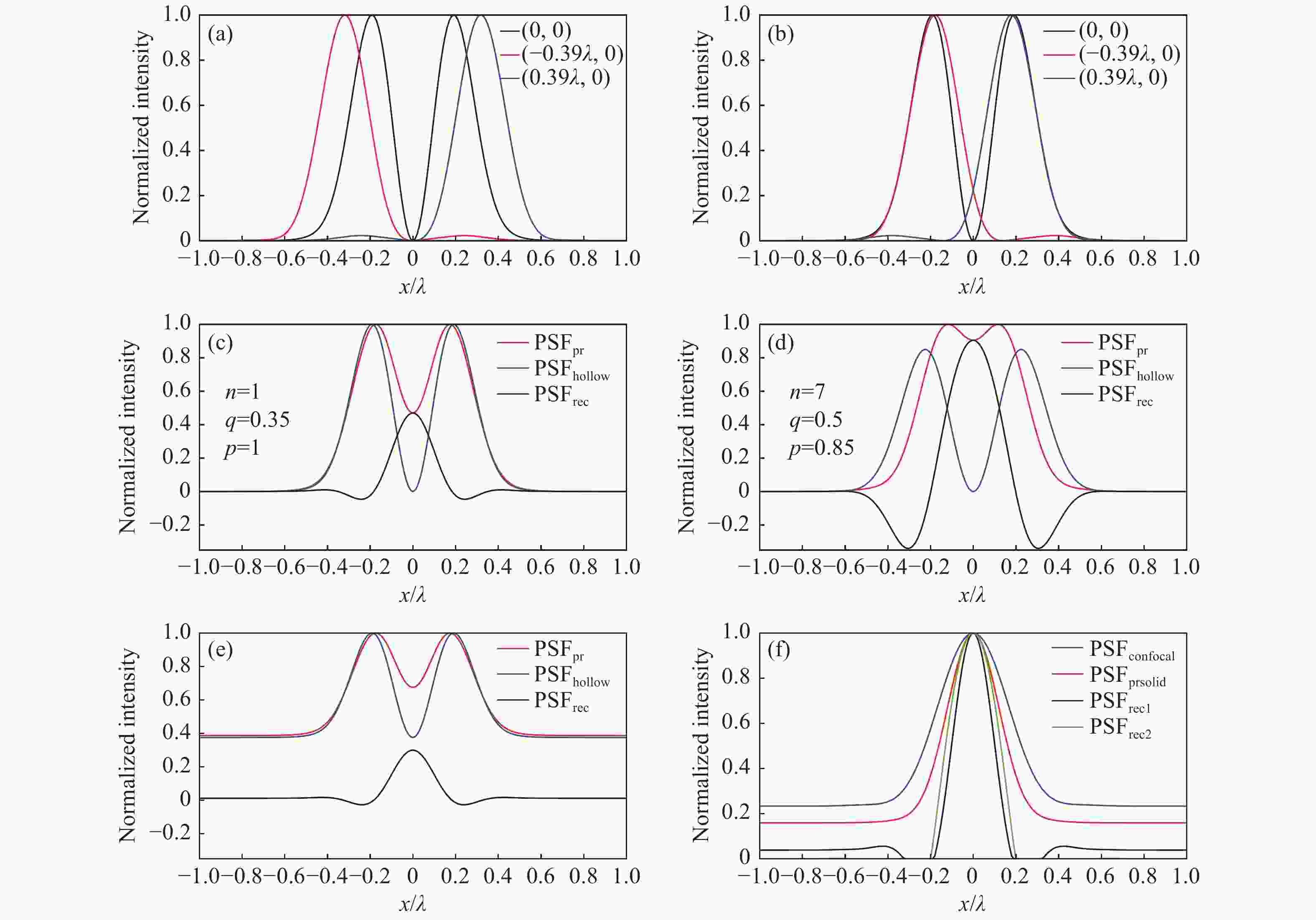
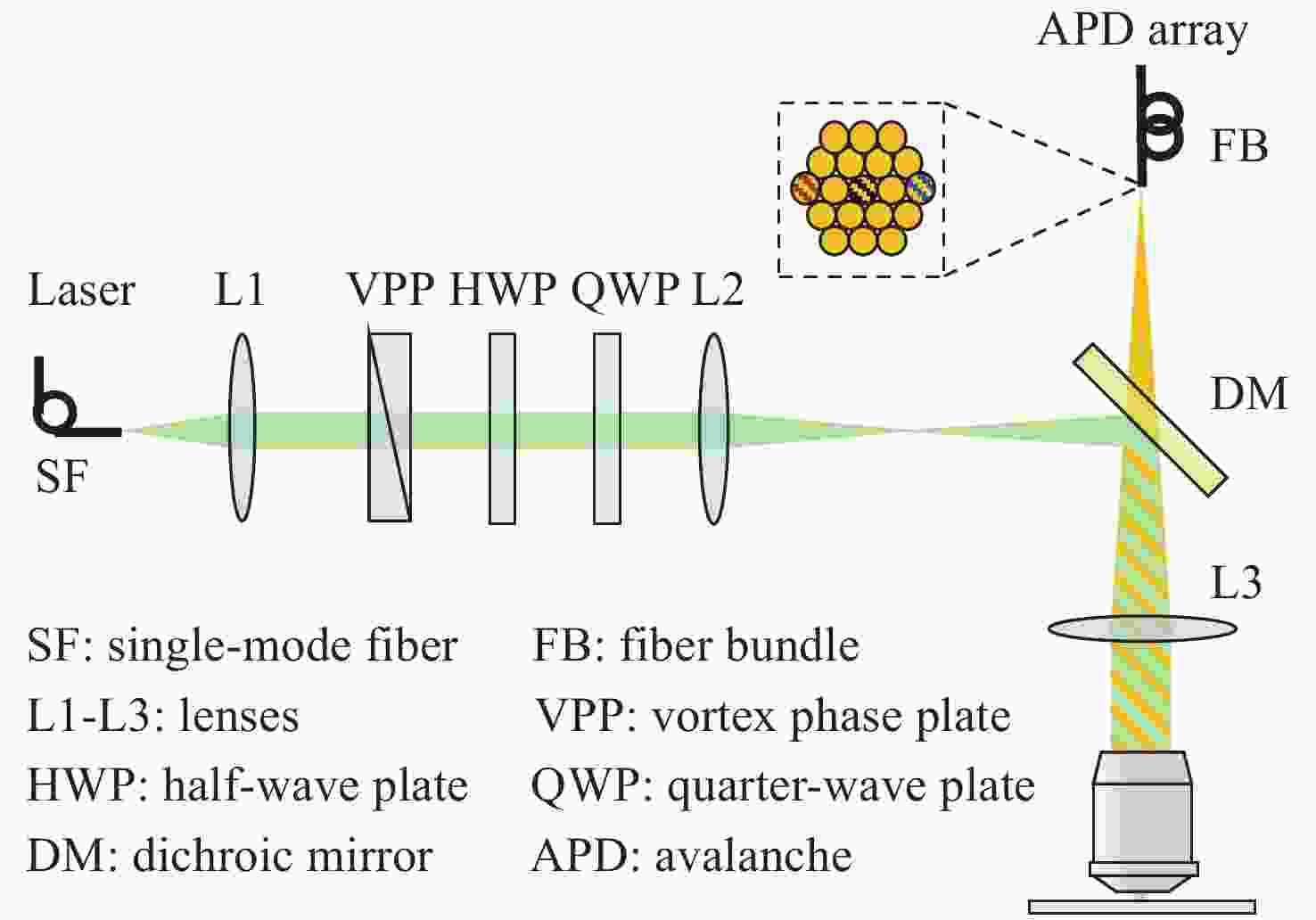
图 2 系统成像模型。(a)黑色、红色、蓝色曲线分别是并行探测的中心通道和左侧、右侧边缘通道的PSF的归一化强度分布;(b)进行光子重组后边缘通道的PSF归一化强度分布,与中心通道外轮廓匹配;(c)n=1,q=0.35,p=1,无噪声时的vFED成像模型,红色、蓝色、黑色曲线分别是光子重组并相加得到的虚拟实心光斑图像PSF、中心通道获得的空心光斑图像PSF、vFED图像PSF;(d)n=7,q=0.5,p=0.85,无噪声时的vFED成像模型,曲线的意义和(c)相同;(e) 考虑背景噪声时的vFED成像模型,各通道包含的背景噪声在该通道总光子数中的占比相同,参数n、q、p与(c)相同;(f)蓝色、红色、黑色、绿色曲线分别是共聚焦PSF、实心光斑光子重组方法PSF、按(c)和(d)的n、q、p参数计算的vFED图像PSF,背景噪声水平与(e)相同
Figure 2. Imaging model of the system. (a) The black, red, and blue curves are the normalized PSFs of the center, left and right edge channels in parallel detection, respectively; (b) the normalized PSFs of the edge channels after photon reassignment, matching the outer contour of the center channel; vFED imaging model without noise at (c) n=1, q=0.35, p=1 and (d) n=7, q=0. 5, p=0.85, the red, blue and black curves are virtual solid spot image PSF obtained by photon reassignment and summation, the hollow spot image PSF obtained from the center channel, and the vFED image PSF, respectively; (e) vFED imaging model when background noise is considered, the background noise contained in each channel accounts for the same proportion of the total number of photons in the channel, and the parameters n, q, p are the same as those in (c); (f) the blue, red, black, and green curves are the confocal PSF, solid spot photon reassignment method PSF, vFED image PSF calculated according to the n, q, p parameters of (c) and (d) , respectively. The background noise level is the same as that in (e)
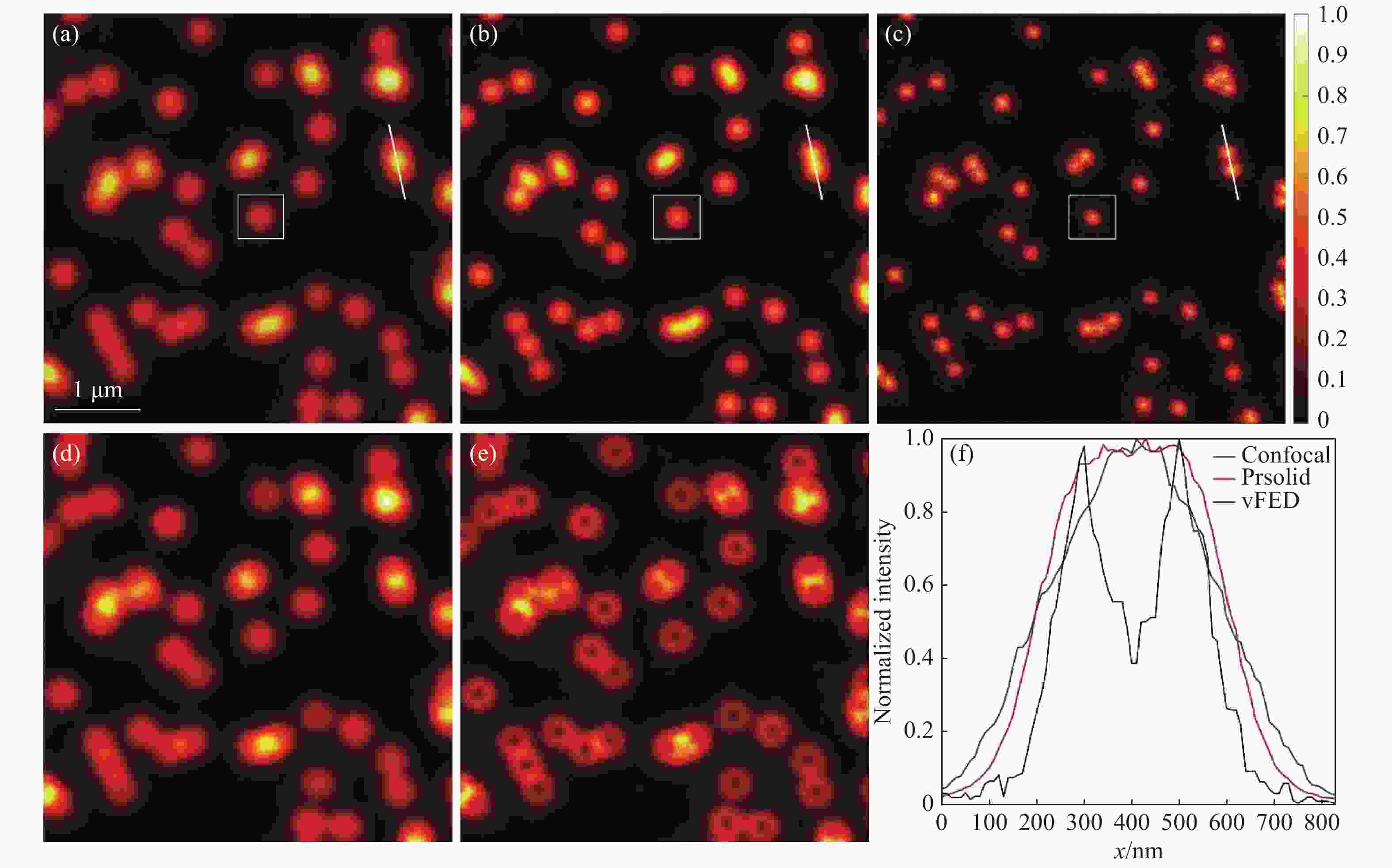
图 3 荧光颗粒成像结果。(a)共聚焦图像;(b)实心光斑光子重组图像;(c)vFED图像;(d)虚拟实心光斑图像;(e)空心光斑图像;(f)图(a)~(c) 中白色截线的归一化强度分布
Figure 3. Fluorescent particle imaging results. (a) Confocal image; (b) solid spot photon reassignment image; (c) vFED image; (d) virtual solid spot image; (e) hollow spot image; (f) normalized intensity of the white truncation line in figures (a)~(c)
-
[1] ABBE E. Beiträge zur theorie des mikroskops und der mikroskopischen wahrnehmung[J]. Archiv für Mikroskopische Anatomie, 1873, 9(1): 413-468. [2] HELL S W, WICHMANN J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy[J]. Optics Letters, 1994, 19(11): 780-782. doi: 10.1364/OL.19.000780 [3] KUANG C F, LI SH, LIU W, et al. Breaking the diffraction barrier using fluorescence emission difference microscopy[J]. Scientific Reports, 2013, 3: 1441. doi: 10.1038/srep01441 [4] BETZIG E, PATTERSON G H, SOUGRAT R, et al. Imaging intracellular fluorescent proteins at nanometer resolution[J]. Science, 2006, 313(5793): 1642-1645. doi: 10.1126/science.1127344 [5] RUST M J, BATES M, ZHUANG X W. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM)[J]. Nature Methods, 2006, 3(10): 793-796. doi: 10.1038/nmeth929 [6] GUSTAFSSON M G L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy[J]. Journal of Microscopy, 2000, 198(2): 82-87. doi: 10.1046/j.1365-2818.2000.00710.x [7] MA Y, KUANG C F, FANG Y, et al. Virtual fluorescence emission difference microscopy based on photon reassignment[J]. Optics Letters, 2015, 40(20): 4627-4630. doi: 10.1364/OL.40.004627 [8] HE M F, HAN Y B, GAN Y H, et al. Dynamic live-cell super-resolution imaging with parallelized fluorescence emission difference microscopy[J]. Optics Communications, 2020, 460: 125087. doi: 10.1016/j.optcom.2019.125087 [9] 张智敏, 黄宇然, 刘少聪, 等. 共路并行荧光辐射差分超分辨显微成像[J]. 中国激光,2021,48(16):1607002. doi: 10.3788/CJL202148.1607002ZHANG ZH M, HUANG Y R, LIU SH C, et al. Common-path parallel fluorescence emission difference super-resolution microscopy[J]. Chinese Journal of Lasers, 2021, 48(16): 1607002. (in Chinese) doi: 10.3788/CJL202148.1607002 [10] LIU SH C, SUN SH Y, KUANG C F, et al. Saturated virtual fluorescence emission difference microscopy based on detector array[J]. Optics Communications, 2017, 395: 45-50. doi: 10.1016/j.optcom.2016.05.024 [11] MÜLLER C B, ENDERLEIN J. Image scanning microscopy[J]. Physical Review Letters, 2010, 104(19): 198101. doi: 10.1103/PhysRevLett.104.198101 [12] SHEPPARD C J R, MEHTA S B, HEINTZMANN R. Superresolution by image scanning microscopy using pixel reassignment[J]. Optics Letters, 2013, 38(15): 2889-2892. doi: 10.1364/OL.38.002889 [13] RICHARDS B, WOLF E. Electromagnetic diffraction in optical systems, Ⅱ. Structure of the image field in an aplanatic system[J]. Proceedings of the Royal Society A:Mathematical,Physical and Engineering Sciences, 1959, 253(1274): 358-379. [14] LIU X, PENG Y F, TU SH J, et al. Generation of arbitrary longitudinal polarization vortices by pupil function manipulation[J]. Advanced Photonics Research, 2021, 2(1): 2000087. doi: 10.1002/adpr.202000087 [15] WANG N, KOBAYASHI T. Numerical study of the subtraction threshold for fluorescence difference microscopy[J]. Optics Express, 2014, 22(23): 28819-28830. doi: 10.1364/OE.22.028819 -





 下载:
下载: