-
摘要:
数字病理凭借其便捷的存储、管理、浏览、传输等特点,为远程病理会诊及联合会诊带来了新契机。然而,显微镜的视场有限,在保证分辨率的前提下,无法兼顾全景成像。全景数字病理的提出弥补了这一缺陷,其在保证分辨率的同时可兼顾全景成像。但单张切片仅能实现单靶点检测,而疾病诊断需同时观测多个靶点的表达情况。近年来,多靶点全景数字病理技术发展迅速,因其在药物研发、临床科研以及基础科研等领域有巨大的应用潜力而广受关注。该系统凭借视场大、颜色多、通量高的特点,可在短时间内原位检测整张组织切片上的多种生物标记物的表达情况,借以识别组织上每个细胞表型、丰度、状态及其相互关系。本文首先梳理了数字病理、全景数字病理以及多靶点全景数字病理的发展过程,并简要介绍发展过程中技术的更新迭代,以及发展多靶点全景数字病理的重要性。然后,分别从生物样本准备、多色光学成像以及图像处理3个部分重点介绍多靶点全景数字病理。接下来,阐述了多靶点全景数字病理在肿瘤微环境与肿瘤分子分型等生物医学领域的应用情况。最后,对多靶点全景数字病理的技术优势、目前面临的挑战及其未来的发展趋势进行了总结。
Abstract:Digital pathology has brought new opportunities for remote pathological consultation and joint consultation owing to its convenient storage, management, browsing and transmission. However, because of the limited field of view of a microscope, panoramic imaging cannot be achieved while ensuring a high resolution. The proposal of panoramic digital pathology makes up for this defect and achieves panoramic imaging while ensuring high resolution. However, a single slice can only detect a single target, and disease diagnosis needs to observe the expression of multi-target at the same time. In recent years, multi-target panoramic digital pathology technology has developed rapidly. It has attracted much attention because of its great application potential in drug research and development, clinical research and basic research. Owing to its large field of view, wide range of colors and high flux, the system can detect the expression of various biomarkers on a whole tissue section in situ in a short time to identify the phenotype, abundance, state, and relationship of each cell. Firstly, this paper reviews the development process of digital pathology, panoramic digital pathology and multi-target panoramic digital pathology, as well as the update and iteration of technology in the development process, and illustrates the importance of developing multi-target panoramic digital pathology. Then, the multi-target panoramic digital pathology is described in detail from three perspectives: biological sample preparation, multi-color imaging system and image processing. Next, the applications of multi-target panoramic digital pathology in biomedical fields, such as tumor microenvironments and tumor molecular typing are described. Finally, the advantages, challenges and future development of multi-target panoramic digital pathology are summarized.
-
表 1 靶点选择原则
Table 1. Target selection principles
疾病 靶点 功能 表达部位 非小细
胞癌[22]CK 一种细胞角蛋白,是上皮来源的肿瘤标志物[23] 主要在上皮细胞中表达的中间丝蛋白[23] CD68 巨噬细胞标记物[24] 存在于骨髓和神经的吞噬细胞[25] Siglec-15 作为免疫调节靶点在肿瘤微环境能
抑制抗原特异性T细胞反应[22]骨髓细胞,人类癌细胞和肿瘤浸润性骨髓细胞[22] 肝癌
组织[26]PD-L1 对抗PD-1/PD-L1治疗反应的预测性生物标志物[27] 骨髓、淋巴、正常上皮细胞和癌症中
组成型表达或诱导的共抑制受体[27]CD68 同上 同上 CD33 髓系细胞分化抗原[28] 主要分布在髓系血细胞[28] CD57 人类自然杀伤细胞标记物[29] 人类自然杀伤细胞和T淋巴细胞[29] CD11b 粘附分子和介导多种配体识别的膜受体[30] 在吞噬细胞、B细胞和T细胞的次要亚群以及自然杀伤细胞上表达[30] CD20 B细胞的表面抗原[31] 在B淋巴细胞上表达[32] 肺癌
组织[33]PD-L1 同上 同上 PD-1 在活化的T细胞中诱导的抑制性受体[34] 活化的T、自然杀伤和B淋巴细胞、巨噬细胞、
树突状细胞和单核细胞上表达[35]CD8 细胞毒性T淋巴细胞标志物[36] 所有细胞毒性T淋巴细胞或杀伤细胞上[36] FoxP3 调节性T细胞的标志性分子[37] 调节性T细胞和正常上皮细胞及多种肿瘤细胞中[37] CD68 同上 同上 CK 同上 同上 头颈癌
组织[38]PD-1 同上 同上 OX40 免疫调节蛋白,主要由T细胞表达[39] 活化的CD4+和CD8+上表达、T细胞上表达以及
许多其他淋巴和非淋巴细胞[39]FoxP3 同上 同上 CD3 T细胞标志物[40] 存在于T细胞表面[40] 乳腺癌
组织[41]CD103 肿瘤浸润调节性T细胞的标志[42] 肠道粘膜上皮内的T细胞群和肠固有层白细胞上表达[42] CD8 同上 同上 表 2 全景数字病理设备产品主要参数
Table 2. The main parameters of panoramic digital pathology equipments
公司 型号 成像模式 切片容量 视场 成像速度 Zeiss Axio Scan.Z1 明场,荧光,偏振(选配) 12或100片(选配) 15 mm × 15 mm 20×明场:240 s/片;
荧光:NAAxioscan 7 明场,荧光,偏振(选配) 100片 10 mm ×10 mm 20×明场:73 s/片
20×荧光:(4通道):323 s/片(ps: non-available, NA) -
[1] GRIFFIN J, TREANOR D. Digital pathology in clinical use: where are we now and what is holding us back?[J]. Histopathology, 2017, 70(1): 134-145. doi: 10.1111/his.12993 [2] BERA K, SCHALPER K A, RIMM D L, et al. Artificial intelligence in digital pathology — new tools for diagnosis and precision oncology[J]. Nature Reviews Clinical Oncology, 2019, 16(11): 703-715. doi: 10.1038/s41571-019-0252-y [3] 黄郑重, 曹良才. 面向高通量的多通道复用数字全息成像技术[J]. 中国光学, doi: 10.37188/CO.2022-0070.HUANG ZH ZH, CAO L C. Multi-channel multiplexing digital holographic imaging for high throughput[J]. Chinese Optics, doi: 10.37188/CO.2022-0070. (in chinese) [4] JAGGI B, POON S S S, MACAULAY C, et al. Imaging system for morphometric assessment of absorption or fluorescence in stained cells[J]. Cytometry, 1988, 9(6): 566-572. doi: 10.1002/cyto.990090609 [5] FURNESS P N. The use of digital images in pathology[J]. The Journal of Pathology, 1997, 183(3): 253-263. doi: 10.1002/(SICI)1096-9896(199711)183:3<253::AID-PATH927>3.0.CO;2-P [6] KRUPINSKI E A. Optimizing the pathology workstation "cockpit": challenges and solutions[J]. Journal of Pathology Informatics, 2010, 1(1): 19. doi: 10.4103/2153-3539.70708 [7] GUZMAN M, JUDKINS A R. Digital pathology: a tool for 21st century neuropathology[J]. Brain Pathology, 2009, 19(2): 305-316. doi: 10.1111/j.1750-3639.2009.00264.x [8] NIAZI M K K, TAVOLARA T E, AROLE V, et al. Identifying tumor in pancreatic neuroendocrine neoplasms from Ki67 images using transfer learning[J]. PloS one, 2018, 13(4): e0195621. doi: 10.1371/journal.pone.0195621 [9] MADABHUSHI A. Digital pathology image analysis: opportunities and challenges[J]. Imaging in Medicine, 2009, 1(1): 7-10. doi: 10.2217/iim.09.9 [10] HUANG Y S, CHOU P R, CHEN H M, et al. One-stage pulmonary nodule detection using 3-D DCNN with feature fusion and attention mechanism in CT image[J]. Computer Methods and Programs in Biomedicine, 2022, 220: 106786. doi: 10.1016/j.cmpb.2022.106786 [11] BIZOT A, KARIMI M, RASSY E, et al. Multicenter evaluation of breast cancer patients’ satisfaction and experience with oncology telemedicine visits during the COVID-19 pandemic[J]. British Journal of Cancer, 2021, 125(11): 1486-1493. doi: 10.1038/s41416-021-01555-y [12] PANTANOWITZ L, VALENSTEIN P N, EVANS A J, et al. Review of the current state of whole slide imaging in pathology[J]. Journal of Pathology Informatics, 2011, 2(1): 36. doi: 10.4103/2153-3539.83746 [13] JAHN S W, PLASS M, MOINFAR F. Digital pathology: advantages, limitations and emerging perspectives[J]. Journal of Clinical Medicine, 2020, 9(11): 3697. doi: 10.3390/jcm9113697 [14] MA B, ZIMMERMANN T, ROHDE M, et al. Use of autostitch for automatic stitching of microscope images[J]. Micron, 2007, 38(5): 492-499. doi: 10.1016/j.micron.2006.07.027 [15] LEGESSE F B, CHERNAVSKAIA O, HEUKE S, et al. Seamless stitching of tile scan microscope images[J]. Journal of Microscopy, 2015, 258(3): 223-232. doi: 10.1111/jmi.12236 [16] DESHPANDE S, MINHAS F, GRAHAM S, et al. SAFRON: stitching across the frontier network for generating colorectal cancer histology images[J]. Medical Image Analysis, 2022, 77: 102337. doi: 10.1016/j.media.2021.102337 [17] JACQUELOT N, SEILLET C, WANG M Y, et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma[J]. Nature immunology, 2021, 22(7): 851-864. doi: 10.1038/s41590-021-00943-z [18] MAYNARD A, MCCOACH C E, ROTOW J K, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing[J]. Cell, 2020, 182(5): 1232-1251.e22. doi: 10.1016/j.cell.2020.07.017 [19] KEREN L, BOSSE M, MARQUEZ D, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging[J]. Cell, 2018, 174(6): 1373-1387.e19. doi: 10.1016/j.cell.2018.08.039 [20] BERRY S, GIRALDO N A, GREEN B F, et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade[J]. Science, 2021, 372(6547): eaba2609. doi: 10.1126/science.aba2609 [21] HENRY N L, HAYES D F. Cancer biomarkers[J]. Molecular Oncology, 2012, 6(2): 140-146. doi: 10.1016/j.molonc.2012.01.010 [22] WANG J, SUN J W, LIU L N, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy[J]. Nature Medicine, 2019, 25(4): 656-666. doi: 10.1038/s41591-019-0374-x [23] TANG K D, KENNY L, PERRY C, et al. The overexpression of salivary cytokeratins as potential diagnostic biomarkers in head and neck squamous cell carcinomas[J]. Oncotarget, 2017, 8(42): 72272-72280. doi: 10.18632/oncotarget.19731 [24] HOLNESS C L, SIMMONS D L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins[J]. Blood, 1993, 81(6): 1607-1613. doi: 10.1182/blood.V81.6.1607.1607 [25] GOTTFRIED E, KUNZ-SCHUGHART L A, WEBER A, et al. Expression of CD68 in non-myeloid cell types[J]. Scandinavian Journal of Immunology, 2008, 67(5): 453-463. doi: 10.1111/j.1365-3083.2008.02091.x [26] LIU CH Q, XU J, ZHOU ZH G, et al. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma[J]. British Journal of Cancer, 2018, 119(1): 80-88. doi: 10.1038/s41416-018-0144-4 [27] KYTHREOTOU A, SIDDIQUE A, MAURI F A, et al. Pd-L1[J]. Journal of Clinical Pathology, 2018, 71(3): 189-194. doi: 10.1136/jclinpath-2017-204853 [28] WALTER R B, APPELBAUM F R, ESTEY E H, et al. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy[J]. Blood, 2012, 119(26): 6198-6208. doi: 10.1182/blood-2011-11-325050 [29] KARED H, MARTELLI S, NG T P, et al. CD57 in human natural killer cells and T-lymphocytes[J]. Cancer Immunology,Immunotherapy, 2016, 65(4): 441-452. doi: 10.1007/s00262-016-1803-z [30] ROSS G D, VĚTVIČKA V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions[J]. Clinical &Experimental Immunology, 1993, 92(2): 181-184. [31] TEDDER T F, ENGEL P. CD20: a regulator of cell-cycle progression of B lymphocytes[J]. Immunology Today, 1994, 15(9): 450-454. doi: 10.1016/0167-5699(94)90276-3 [32] BOROSS P, LEUSEN J H W. Mechanisms of action of CD20 antibodies[J]. American Journal of Cancer Research, 2012, 2(6): 676-690. [33] FORDE P M, CHAFT J E, SMITH K N, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer[J]. New England Journal of Medicine, 2018, 378(21): 1976-1986. doi: 10.1056/NEJMoa1716078 [34] PATSOUKIS N, WANG Q, STRAUSS L, et al. Revisiting the PD-1 pathway[J]. Science Advances, 2020, 6(38): eabd2712. doi: 10.1126/sciadv.abd2712 [35] AHMADZADEH M, JOHNSON L A, HEEMSKERK B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired[J]. Blood, 2009, 114(8): 1537-1544. doi: 10.1182/blood-2008-12-195792 [36] COLE D K, GAO G F. CD8: adhesion molecule, co-receptor and immuno-modulator[J]. Cellular &Molecular Immunology, 2004, 1(2): 81-88. [37] TRIULZI T, TAGLIABUE E, BALSARI A, et al. FOXP3 expression in tumor cells and implications for cancer progression[J]. Journal of Cellular Physiology, 2013, 228(1): 30-35. doi: 10.1002/jcp.24125 [38] MONTLER R, BELL R B, THALHOFER C, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer[J]. Clinical &Translational Immunology, 2016, 5(4): e70. [39] CROFT M, SO T, DUAN W, et al. The significance of OX40 and OX40L to T-cell biology and immune disease[J]. Immunological Reviews, 2009, 229(1): 173-191. doi: 10.1111/j.1600-065X.2009.00766.x [40] DONG D, ZHENG L Q, LIN J Q, et al. Structural basis of assembly of the human T cell receptor-CD3 complex[J]. Nature, 2019, 573(7775): 546-552. doi: 10.1038/s41586-019-1537-0 [41] SAVAS P, VIRASSAMY B, YE CH ZH, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis[J]. Nature Medicine, 2018, 24(7): 986-993. doi: 10.1038/s41591-018-0078-7 [42] ANZ D, MUELLER W, GOLIC M, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells[J]. International Journal of Cancer, 2011, 129(10): 2417-2426. doi: 10.1002/ijc.25902 [43] CARLOS F M. Pancreatic cancer: investigation of the prognostic significance of tumor immunophenotype and establishment of a novel ex-vivo tissue slice culture system for drug sensitivity testing[D]. Stockholm: Karolinska Institutet, 2021. [44] KANG C, QIAO Y, LI G, et al. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials[J]. Scientific Reports, 2016, 6: 28798. doi: 10.1038/srep28798 [45] MERZ F, GAUNITZ F, DEHGHANI F, et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments[J]. Neuro-Oncology, 2013, 15(6): 670-681. doi: 10.1093/neuonc/not003 [46] ZHANG K, HAO CH Y, MAN B Y, et al. Diagnosis of liver cancer based on tissue slice surface enhanced Raman spectroscopy and multivariate analysis[J]. Vibrational Spectroscopy, 2018, 98: 82-87. doi: 10.1016/j.vibspec.2018.07.010 [47] HAEHNEL S, REICHE K, LOEFFLER D, et al. Deep sequencing and automated histochemistry of human tissue slice cultures improve their usability as preclinical model for cancer research[J]. Scientific Reports, 2019, 9(1): 19961. doi: 10.1038/s41598-019-56509-5 [48] INAMURA K. Update on immunohistochemistry for the diagnosis of lung cancer[J]. Cancers, 2018, 10(3): 72. doi: 10.3390/cancers10030072 [49] NASR S H, FIDLER M E, SAID S M. Paraffin immunofluorescence: a valuable ancillary technique in renal pathology[J]. Kidney International Reports, 2018, 3(6): 1260-1266. doi: 10.1016/j.ekir.2018.07.008 [50] BRUNNSTRÖM H, JOHANSSON A, WESTBOM-FREMER S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability[J]. Modern Pathology, 2017, 30(10): 1411-1421. doi: 10.1038/modpathol.2017.59 [51] MEYERHOLZ D K, BECK A P. Principles and approaches for reproducible scoring of tissue stains in research[J]. Laboratory Investigation, 2018, 98(7): 844-855. doi: 10.1038/s41374-018-0057-0 [52] TAN W C C, NERURKAR S N, CAI H Y, et al. Overview of multiplex immunohistochemistry /immunofluorescence techniques in the era of cancer immunotherapy[J]. Cancer Communications, 2020, 40(4): 135-153. doi: 10.1002/cac2.12023 [53] ZRAZHEVSKIY P, GAO X. Quantum dot imaging platform for single-cell molecular profiling[J]. Nature Communication, 2013, 4: 1619. doi: 10.1038/ncomms2635 [54] GOLTSEV Y, SAMUSIK N, KENNEDY-DARLING J, et al. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging[J]. Cell, 2018, 174(4): 968-981.e15. doi: 10.1016/j.cell.2018.07.010 [55] REMARK R, MERGHOUB T, GRABE N, et al. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide[J]. Science Immunology, 2016, 1(1): aaf6925. [56] ZHANG W J, HUBBARD A, JONES T, et al. Fully automated 5-plex fluorescent immunohistochemistry with tyramide signal amplification and same species antibodies[J]. Laboratory Investigation, 2017, 97(7): 873-885. doi: 10.1038/labinvest.2017.37 [57] GERDES M J, SEVINSKY C J, SOOD A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(29): 11982-11987. doi: 10.1073/pnas.1300136110 [58] JEŽ M, BAS T, VEBER M, et al. The hazards of DAPI photoconversion: effects of dye, mounting media and fixative, and how to minimize the problem[J]. Histochemistry and Cell Biology, 2013, 139(1): 195-204. doi: 10.1007/s00418-012-1039-8 [59] KOBAYASHI H, CHOYKE P L, OGAWA M. Monoclonal antibody-based optical molecular imaging probes; considerations and caveats in chemistry, biology and pharmacology[J]. Current Opinion in Chemical Biology, 2016, 33: 32-38. doi: 10.1016/j.cbpa.2016.05.015 [60] GEBHARDT C, LEHMANN M, REIF M M, et al. Molecular and spectroscopic characterization of green and red cyanine fluorophores from the alexa fluor and AF series[J]. ChemPhysChem, 2021, 22(15): 1566-1583. doi: 10.1002/cphc.202000935 [61] SARKAR P, SRIDHARAN S, LUCHOWSKI R, et al. Photophysical properties of a new DyLight 594 dye[J]. Journal of Photochemistry and Photobiology B:Biology, 2010, 98(1): 35-39. doi: 10.1016/j.jphotobiol.2009.10.005 [62] WANG Y X, BAI ZH H, WANG Q, et al. Experimental investigations on fluorescence excitation and depletion of carbon dots[J]. Journal of Fluorescence, 2017, 27(4): 1435-1441. doi: 10.1007/s10895-017-2082-6 [63] BATES M, HUANG B, DEMPSEY G T, et al. Multicolor super-resolution imaging with photo-switchable fluorescent probes[J]. Science, 2007, 317(5845): 1749-1753. doi: 10.1126/science.1146598 [64] ZHANG Y D, SCHROEDER L K, LESSARD M D, et al. Nanoscale subcellular architecture revealed by multicolor three-dimensional salvaged fluorescence imagingimaging[J]. Nature Methods, 2020, 17(2): 225-231. doi: 10.1038/s41592-019-0676-4 [65] DEMPSEY G T, VAUGHAN J C, CHEN K H, et al. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging[J]. Nature Methods, 2011, 8(12): 1027-1036. doi: 10.1038/nmeth.1768 [66] MATHEW S K, BAYANNA A R, TIWARY A R, et al. First observations from the multi-application solar telescope (MAST) narrow-band imager[J]. Solar Physics, 2017, 292(8): 106. doi: 10.1007/s11207-017-1127-y [67] PISANI M, ZUCCO M. Compact imaging spectrometer combining Fourier transform spectroscopy with a Fabry-Perot interferometer[J]. Optics Express, 2009, 17(10): 8319-8331. doi: 10.1364/OE.17.008319 [68] SINCLAIR M B, TIMLIN J A, HAALAND D M, et al. Design, construction, characterization, and application of a hyperspectral microarray scanner[J]. Applied Optics, 2004, 43(10): 2079-2088. doi: 10.1364/AO.43.002079 [69] MLODZIANOSKI M J, CURTHOYS N M, GUNEWARDENE M S, et al. Super-resolution imaging of molecular emission spectra and single molecule spectral fluctuations[J]. PloS one, 2016, 11(3): e0147506. doi: 10.1371/journal.pone.0147506 [70] JAHR W, SCHMID B, SCHMIED C, et al. Hyperspectral light sheet microscopy[J]. Nature communications, 2015, 6: 7990. doi: 10.1038/ncomms8990 [71] YAMASHITA T, KINOSHITA H, SAKAGUCHI T, et al. Objective tumor distinction in 5-aminolevulinic acid-based endoscopic photodynamic diagnosis, using a spectrometer with a liquid crystal tunable filter[J]. Annals of Translational Medicine, 2020, 8(5): 178. doi: 10.21037/atm.2020.01.108 [72] XU Z F, ZHAO H J, JIA G R, et al. Optical schemes of super-angular AOTF-based imagers and system response analysis[J]. Optics Communications, 2021, 498: 127204. doi: 10.1016/j.optcom.2021.127204 [73] LIU CH H, CHANG Y C, NORRIS T B, et al. Graphene photodetectors with ultra-broadband and high responsivity at room temperature[J]. Nature Nanotechnology, 2014, 9(4): 273-278. doi: 10.1038/nnano.2014.31 [74] VALM A M, COHEN S, LEGANT W R, et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome[J]. Nature, 2017, 546(7656): 162-167. doi: 10.1038/nature22369 [75] GHAZNAVI F, EVANS A, MADABHUSHI A, et al. Digital imaging in pathology: whole-slide imaging and beyond[J]. Annual Review of Pathology-Mechanisms of Disease, 2013, 8: 331-359. doi: 10.1146/annurev-pathol-011811-120902 [76] PANTANOWITZ L, DICKINSON K, EVANS A J, et al. American Telemedicine Association clinical guidelines for telepathology[J]. Journal of Pathology Informatics, 2014, 5(1): 39. doi: 10.4103/2153-3539.143329 [77] FARAHANI N, PARWANI A V, PANTANOWITZ L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives[J]. Pathology and Laboratory Medicine International, 2015, 7: 23-33. [78] ROJO M G, GARCíA G B, MATEOS C P, et al. Critical comparison of 31 commercially available digital slide systems in pathology[J]. International Journal of Surgical Pathology, 2006, 14(4): 285-305. doi: 10.1177/1066896906292274 [79] LIAO J, BIAN L H, BIAN Z CH, et al. Single-frame rapid autofocusing for brightfield and fluorescence whole slide imaging[J]. Biomedical Optics Express, 2016, 7(11): 4763-4768. doi: 10.1364/BOE.7.004763 [80] HAMAMATSU. NanoZoomer[EB/OL]. [2022-11-08].https://www.hamamatsu.com.cn/cn/zh-cn/product/life-science-and-medical-systems/digital-slide-scanner/C13140-01.html. [81] 3DHISTECH. Pannoramic 250 Flash III[EB/OL]. [2022-11-08].https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-250-flash-iii/. [82] AFEWORK A, BEYNON M D, BUSTAMANTE F, et al. . Digital dynamic telepathology--the Virtual Microscope[C]. Proceedings American Medical Informatics Association Annual Symposium, AMIA, 1998: 912-916. [83] AMIN W, SRINTRAPUN S J, PARWANI A V. Automated whole slide imaging[J]. Expert Opinion on Medical Diagnostics, 2008, 2(10): 1173-1181. doi: 10.1517/17530059.2.10.1173 [84] OLYMPUS. VS200[EB/OL]. [2022-05-05]. https://www.olympus-lifescience.com.cn/zh/downloads/detail/?0[downloads][id]=847252676. [85] ZEISS. Axio Scan. Z1[EB/OL]. [2022-11-08].https://www.zeiss.com/microscopy/us/local/zen-knowledge-base-home/zen-knowledge-base-axio-scan.html. [86] PLATTARD D, SORET M, TROCCAZ J, et al. Patient set-up using portal images: 2D/2D image registration using mutual information[J]. Computer Aided Surgery, 2000, 5(4): 246-262. doi: 10.3109/10929080009148893 [87] BAY H, ESS A, TUYTELAARS T, et al. Speeded-up robust features (SURF)[J]. Computer Vision and Image Understanding, 2008, 110(3): 346-359. doi: 10.1016/j.cviu.2007.09.014 [88] GHOSH D, KAABOUCH N. A survey on image mosaicing techniques[J]. Journal of Visual Communication and Image Representation, 2016, 34: 1-11. doi: 10.1016/j.jvcir.2015.10.014 [89] 中国政府采购网. 南方医科大学南方医院采购医用设备招标项目[EB/OL]. (2022-03-02)[2022-11-08]. http://www.ccgp.gov.cn/cggg/dfgg/gkzb/202203/t20220302_17632544.htm.Government Procurement Network. Procurement Medical Equipment Bidding Project of Southern Medical University Southern Hospital [EB/OL]. (2022-03-02)[2022-11-08]. http://www.ccgp.gov.cn/cggg/dfgg/gkzb/202203/t20220302_17632544.htm. [90] STATHONIKOS N, NGUYEN T Q, SPOTO C P, et al. Being fully digital: perspective of a Dutch academic pathology laboratory[J]. Histopathology, 2019, 75(5): 621-635. doi: 10.1111/his.13953 [91] NIAZI M K K, PARWANI A V, GURCAN M N. Digital pathology and artificial intelligence[J]. The Lancet Oncology, 2019, 20(5): e253-e261. doi: 10.1016/S1470-2045(19)30154-8 [92] SALVI M, MOLINARO L, METOVIC J, et al. Fully automated quantitative assessment of hepatic steatosis in liver transplants[J]. Computers in Biology and Medicine, 2020, 123: 103836. doi: 10.1016/j.compbiomed.2020.103836 [93] BÁNDI P, VAN DE LOO R, INTEZAR M, et al. . Comparison of different methods for tissue segmentation in histopathological whole-slide images[C]. 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), IEEE, 2017: 591-595. [94] WU H, PHAN J H, BHATIA A K, et al. . Detection of blur artifacts in histopathological whole-slide images of endomyocardial biopsies[C]. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, 2015: 727-730. [95] SALVI M, ACHARYA U R, MOLINARI F, et al. The impact of pre- and post-image processing techniques on deep learning frameworks: A comprehensive review for digital pathology image analysis[J]. Computers in Biology and Medicine, 2021, 128: 104129. doi: 10.1016/j.compbiomed.2020.104129 [96] ARESTA G, ARAÚJO T, KWOK S, et al. BACH: Grand challenge on breast cancer histology images[J]. Medical Image Analysis, 2019, 56: 122-139. doi: 10.1016/j.media.2019.05.010 [97] CIREŞAN D C, GIUSTI A, GAMBARDELLA L M, et al. . Mitosis detection in breast cancer histology images with deep neural networks[C]. 16th International Conference on Medical Image Computing and Computer-Assisted Intervention, Springer, 2013: 411-418. [98] CHEN T, CHEFD’HOTEL C. Deep Learning Based Automatic Immune Cell Detection for Immunohistochemistry Images[C]. 5th International Workshop on Machine Learning in Medical Imaging, Springer, 2014: 17-24. [99] 黄国虹, 李智伟, 王昌敏. 不同免疫表型多发性骨髓瘤患者外周血淋巴细胞亚群分析[J]. 肿瘤研究与临床,2018,30(3):176-179. doi: 10.3760/cma.j.issn.1006-9801.2018.03.008HUANG G H, LI ZH W, WANG CH M. Analysis of peripheral blood lymphocyte subsets in multiple myeloma patients with different immunophenotypes[J]. Cancer Research and Clinic, 2018, 30(3): 176-179. (in Chinese) doi: 10.3760/cma.j.issn.1006-9801.2018.03.008 [100] KOELZER V H, GISLER A, HANHART J C, et al. Digital image analysis improves precision of PD-L1 scoring in cutaneous melanoma[J]. Histopathology, 2018, 73(3): 397-406. doi: 10.1111/his.13528 [101] SUN D CH, GUAN X N, MORAN A E, et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data[J]. Nature Biotechnology, 2022, 40(4): 527-538. doi: 10.1038/s41587-021-01091-3 [102] HARDER N, SCHöNMEYER R, NEKOLLA K, et al. Automatic discovery of image-based signatures for ipilimumab response prediction in malignant melanoma[J]. Scientific Reports, 2019, 9(1): 7449. doi: 10.1038/s41598-019-43525-8 [103] HUANG W, HENNRICK K, DREW S. A colorful future of quantitative pathology: validation of Vectra technology using chromogenic multiplexed immunohistochemistry and prostate tissue microarrays[J]. Human Pathology, 2013, 44(1): 29-38. doi: 10.1016/j.humpath.2012.05.009 [104] OGUEJIOFOR K, HALL J, SLATER C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma[J]. British Journal of Cancer, 2015, 113(6): 886-893. doi: 10.1038/bjc.2015.277 [105] FAILMEZGER H, MURALIDHAR S, RULLAN A, et al. Topological tumor graphs: a graph-based spatial model to infer stromal recruitment for immunosuppression in melanoma histology[J]. Cancer Research, 2020, 80(5): 1199-1209. doi: 10.1158/0008-5472.CAN-19-2268 [106] BARUA S, FANG P, SHARMA A, et al. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer[J]. Lung Cancer, 2018, 117: 73-79. doi: 10.1016/j.lungcan.2018.01.022 [107] ISHII G, OCHIAI A, NERI S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment[J]. Advanced Drug Delivery Reviews, 2016, 99: 186-196. doi: 10.1016/j.addr.2015.07.007 [108] WOOD S L, PERNEMALM M, CROSBIE P A, et al. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets[J]. Cancer Treatment Reviews, 2014, 40(4): 558-566. doi: 10.1016/j.ctrv.2013.10.001 [109] SCHULZ D, ZANOTELLI V R T, FISCHER J R, et al. Simultaneous multiplexed imaging of mRNA and proteins with subcellular resolution in breast cancer tissue samples by mass cytometry[J]. Cell Systems, 2018, 6(1): 25-36.e5. doi: 10.1016/j.cels.2017.12.001 [110] CASCONE T, WILLIAM W N, WEISSFERDT A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial[J]. Nature Medicine, 2021, 27(3): 504-514. doi: 10.1038/s41591-020-01224-2 [111] OBRADOVIC A, CHOWDHURY N, HAAKE S M, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages[J]. Cell, 2021, 184(11): 2988-3005.e16. doi: 10.1016/j.cell.2021.04.038 [112] 赵晶, 付丽. 乳腺癌的分子分型[J]. 中华乳腺病杂志(电子版),2009,3(2):195-203.ZHAO J, FU L. Molecular classification of breast cancer[J]. Chinese Journal of Breast Diseases (Electronic Edition) , 2009, 3(2): 195-203. (in Chinese) [113] HU ZH Y, FAN CH, OH D S, et al. The molecular portraits of breast tumors are conserved across microarray platforms[J]. BMC Genomics, 2006, 7: 96. doi: 10.1186/1471-2164-7-96 [114] HELMINK B A, REDDY S M, GAO J J, et al. B cells and tertiary lymphoid structures promote immunotherapy response[J]. Nature, 2020, 577(7791): 549-555. doi: 10.1038/s41586-019-1922-8 [115] PARRA E R, FRANCISCO-CRUZ A, WISTUBA I I. State-of-the-art of profiling immune contexture in the era of multiplexed staining and digital analysis to study paraffin tumor tissues[J]. Cancers, 2019, 11(2): 247. doi: 10.3390/cancers11020247 [116] TAUBE J M, AKTURK G, ANGELO M, et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation[J]. Journal for Immunotherapy of Cancer, 2020, 8(1): e000155. doi: 10.1136/jitc-2019-000155 [117] CHEN K, YAN R, XIANG L M, et al. Excitation spectral microscopy for highly multiplexed fluorescence imaging and quantitative biosensing[J]. Light:Science &Applications, 2021, 10(1): 97. -





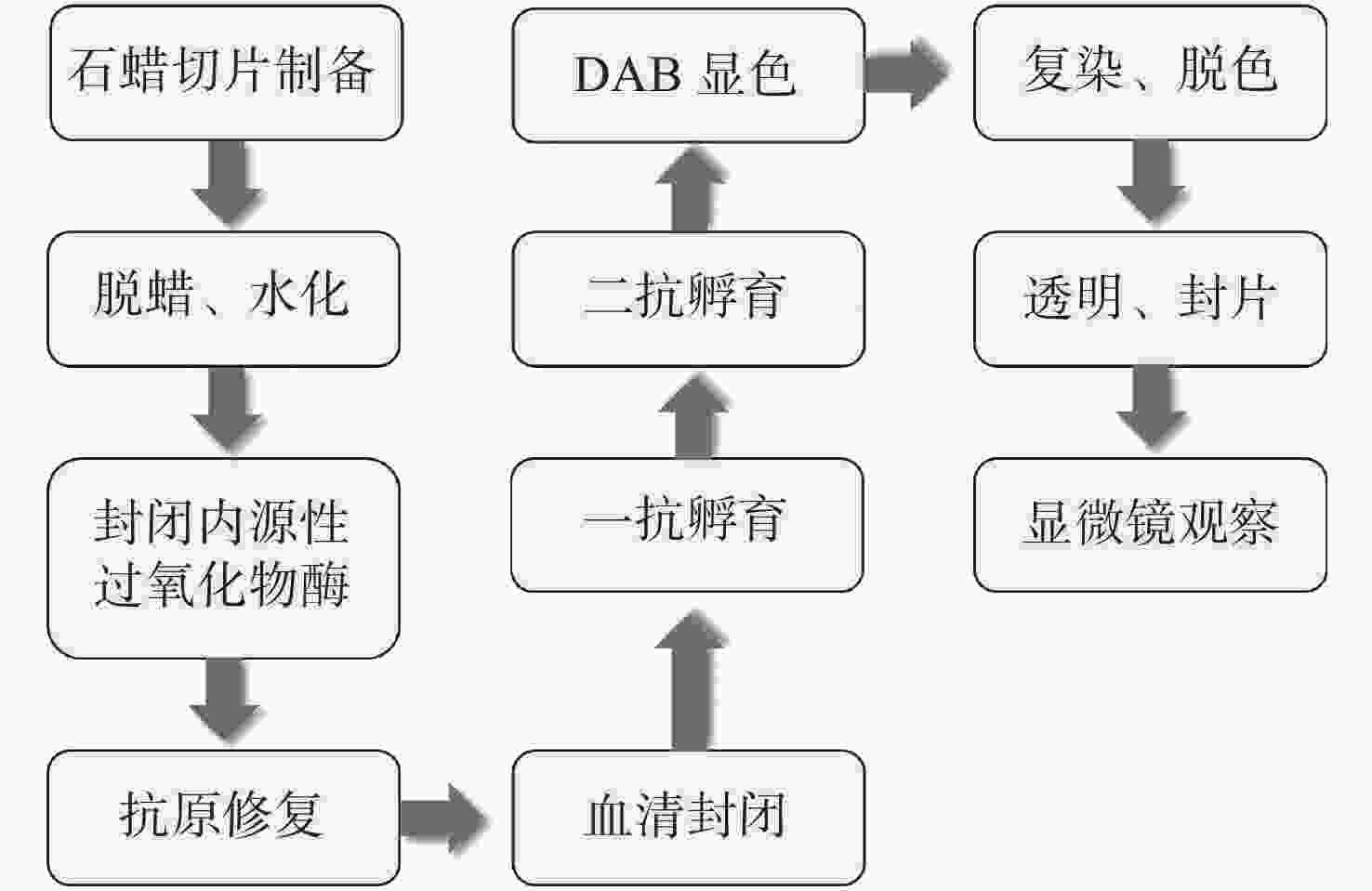
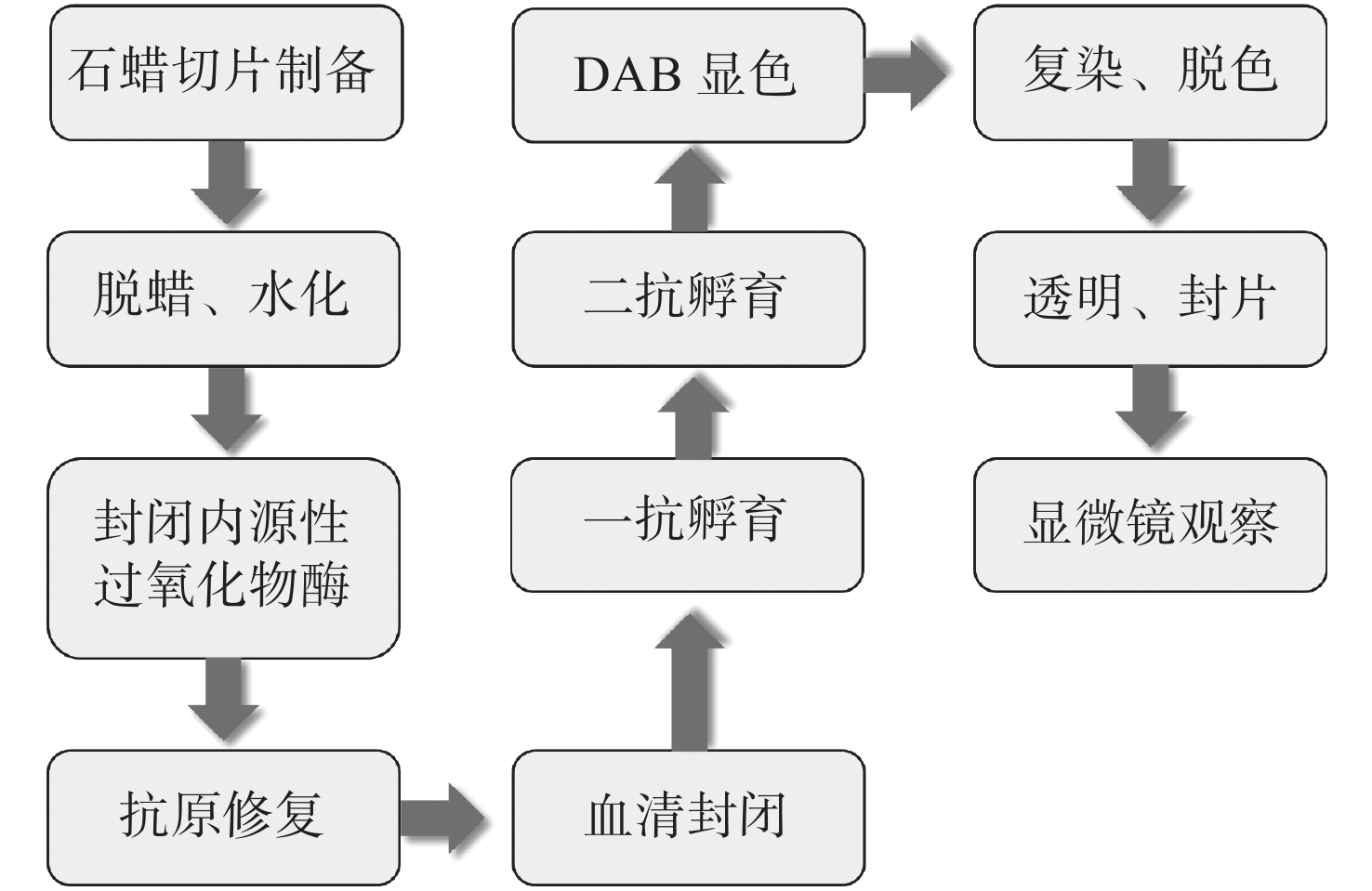
 下载:
下载: