-
摘要:
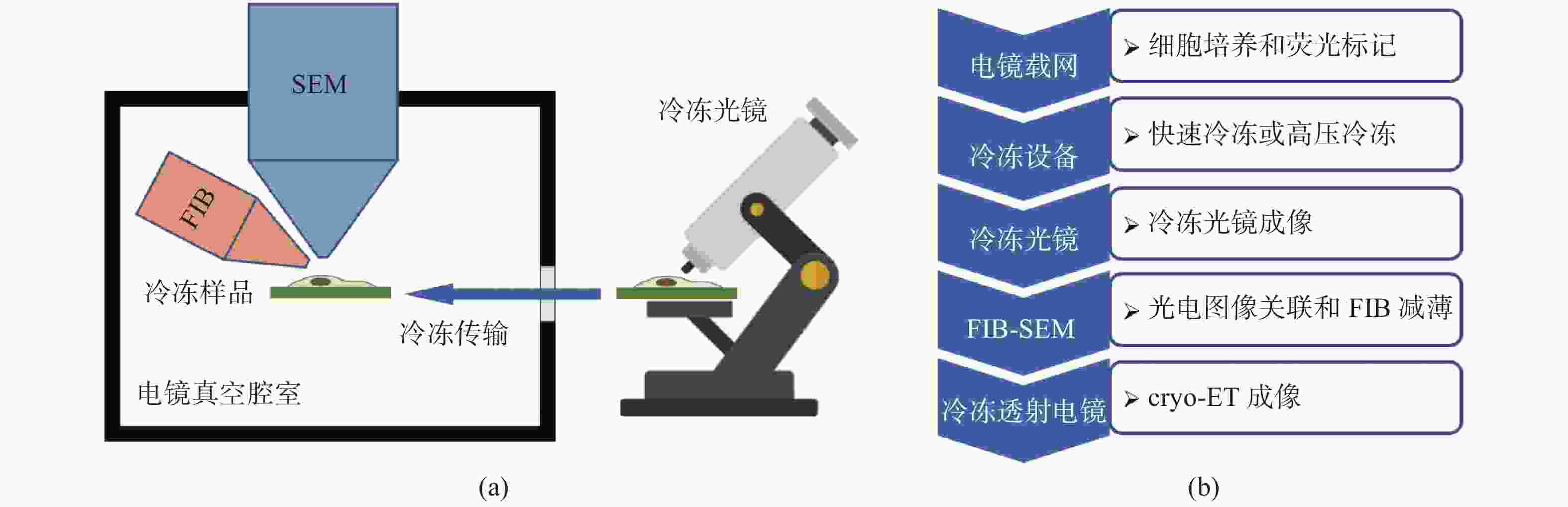
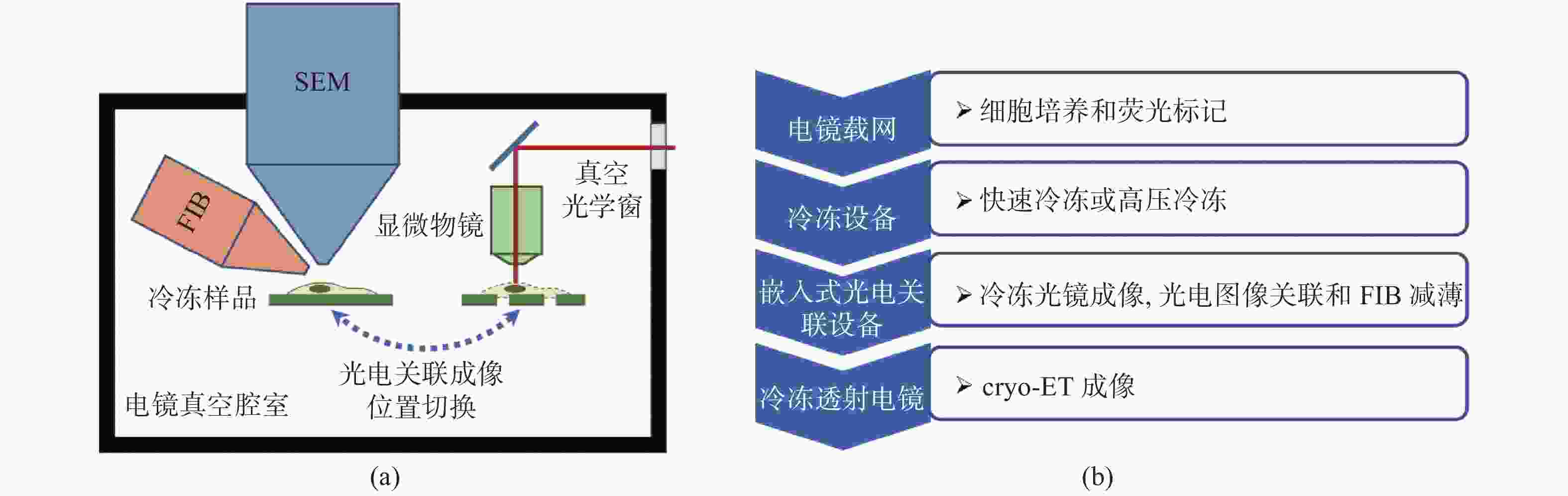
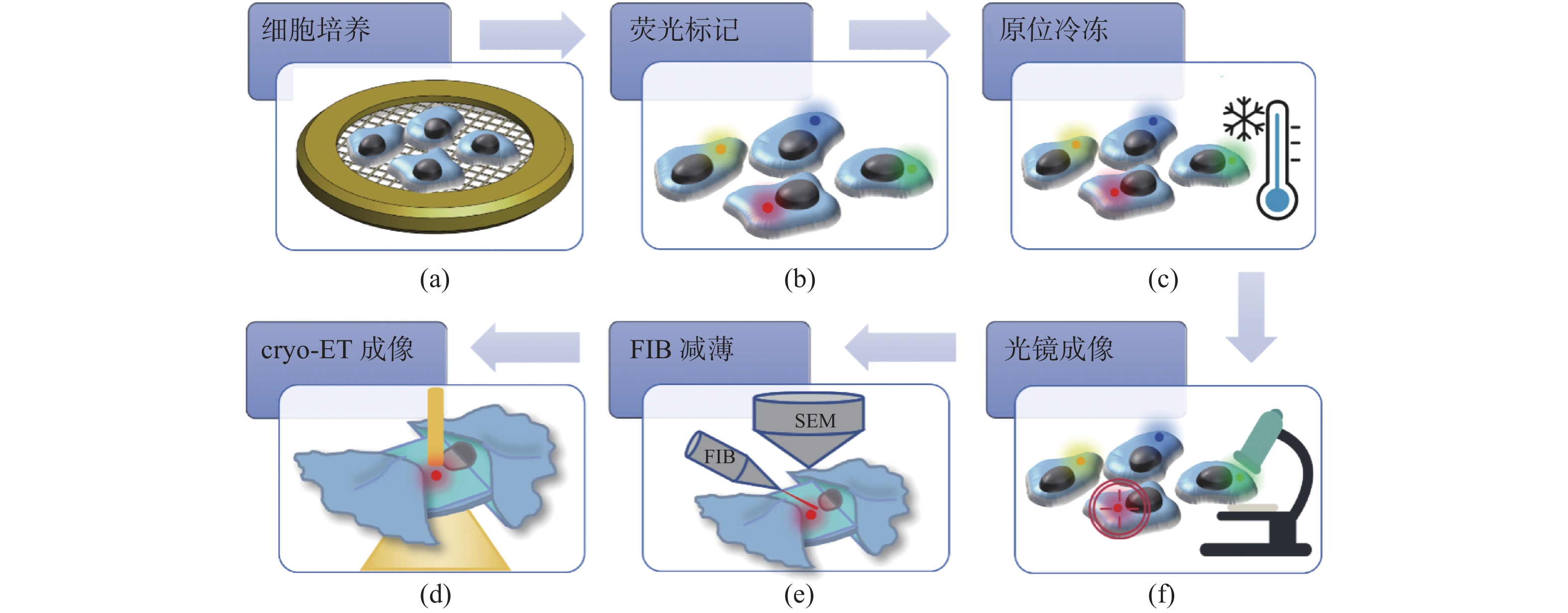
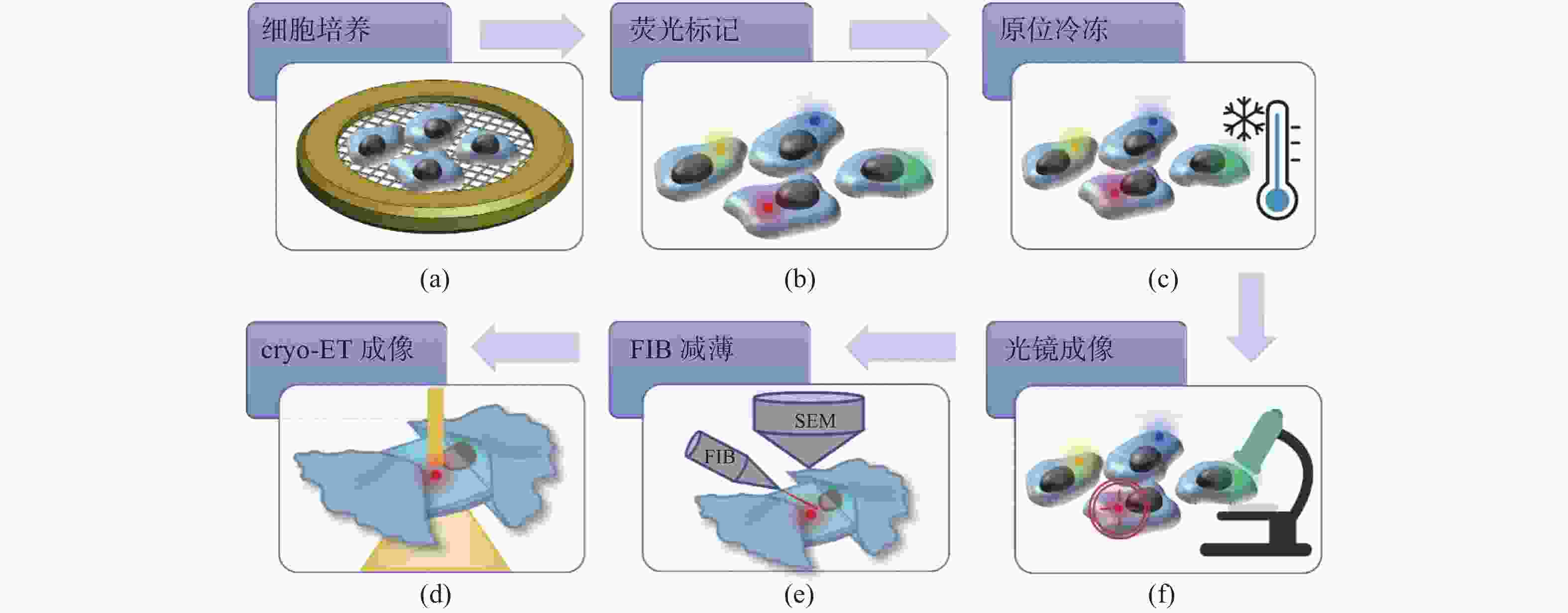
冷冻电子断层扫描成像(cryo-ET)是细胞原位解析生物大分子结构的核心技术,cryo-ET的样品厚度需要小于300 nm,冷冻样品聚焦离子束减薄(FIB)是样品制备流程中的必要环节。当前,FIB存在难以迅速精确定位目标区域的问题,原位冷冻光电关联技术(cryo-CLEM)是一项新兴的技术,对原位冷冻样品分别进行冷冻光镜成像和电镜成像,结合了荧光成像的定位优势和电镜成像的分辨率优势,通过将光镜和电镜图像进行配准,指导FIB对原位冷冻样品减薄,能够极大地提高cryo-ET的样品制备效率。本文介绍了cryo-CLEM中的原位冷冻技术和光电关联成像技术的最新进展和应用情况,重点讨论了超分辨cryo-CLEM成像技术以及嵌入式cryo-CLEM技术,分析了各种方法的优缺点和适用范围,并对cryo-CLEM技术当前面临的主要限制和未来的发展方向进行了展望。
-
关键词:
- 光电关联成像 /
- 荧光导航聚焦离子束减薄 /
- 光学超分辨
Abstract:Cryo-electron tomography (cryo-ET) has become a cutting-edge technology in life sciences for the investigation of protein complexes directly in their natural state. In cryo-ET, the sample’s thickness must be less than 300 nm and the target molecule must be within the lamella, which is prepared by cryo-Focus Iron Beam (FIB) milling. In order to precisely navigate molecules and to improve the efficiency of sample preparation, cryo-Correlative Light and Electron Microscopy (cryo-CLEM) has been introduced to perform in-situ imaging on the frozen samples. The cryo-CLEM combines the localization advantages of fluorescence imaging with the resolution advantages of electron microscopy. By registering images of light and electrons, frozen samples can be thinned by FIB milling, so the efficiency of cryo-ET sample preparation can be improved. In this paper, we review the latest progress and applications of cryo-CLEM technologies, with a particular focus on super-resolution cryo-CLEM imaging and integrated cryo-CLEM. The advantages and limitations of various methodologies, as well as their application scope, are discussed. A discussion on cryo-CLEM's limitations and potential directions for its future development are also presented.
-
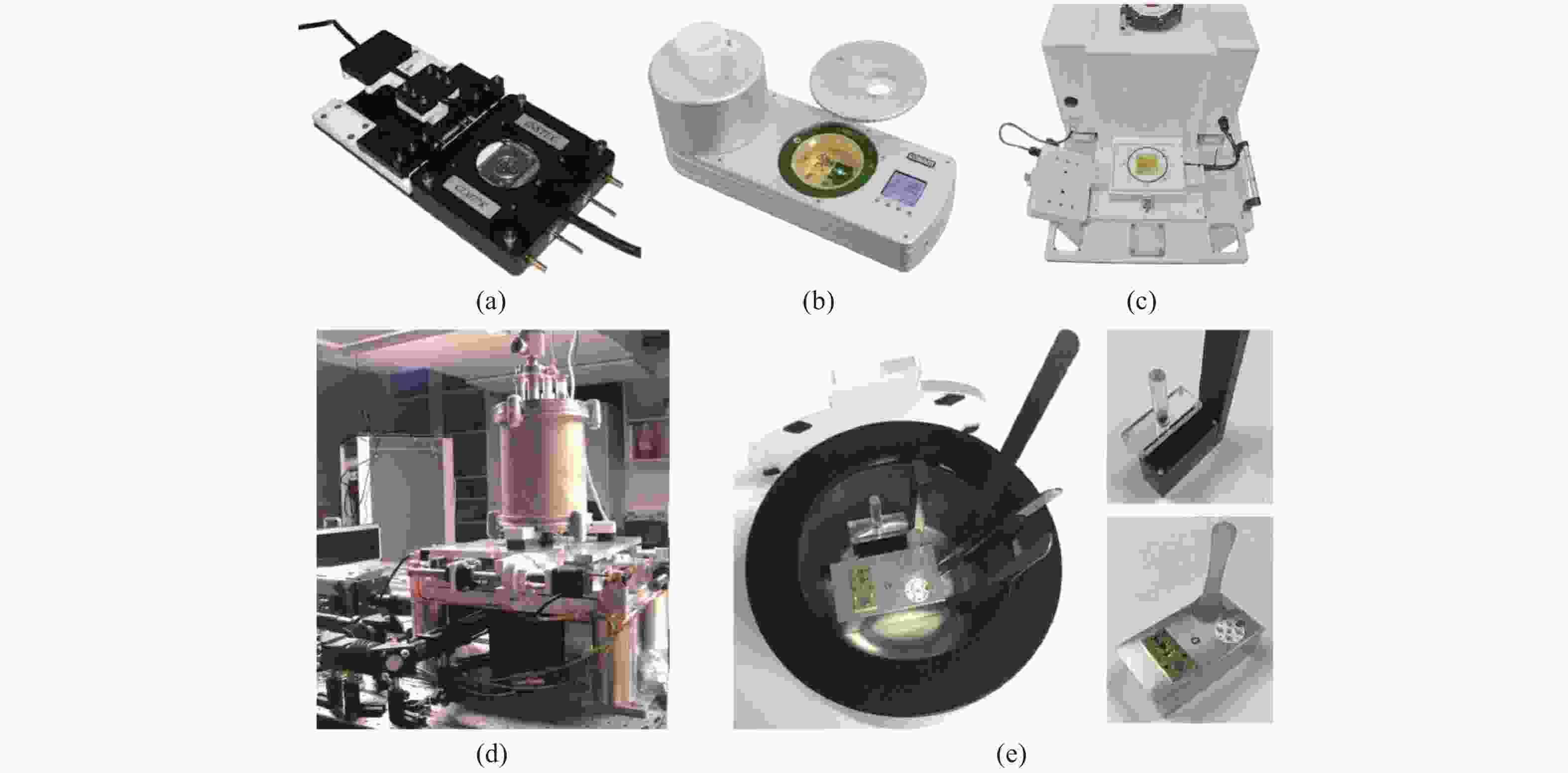
图 3 几种商用冷台和冷台样机实物图。(a)Instec公司的CLM77K;(b)Linkam公司的CMS196;(c)FEI公司的CorrSight;(d)Li等人提出的高稳定性冷台[19];(e)徐涛组提出的高稳定冷台[20]
Figure 3. Commercial cryo stages and prototypes. (a) CLM77K from Instec; (b) CMS196 from Linkam; (c) CorrSight from FEI; (d) Cryo stage proposed by Li[19]; (e) Cryo stage proposed by Xu[20]
-
[1] HYLTON R K, SWULIUS M T. Challenges and triumphs in cryo-electron tomography[J]. Iscience, 2021, 24(9): 102959. doi: 10.1016/j.isci.2021.102959 [2] MARTYNOWYCZ M W, CLABBERS M T B, UNGE J, et al. Benchmarking the ideal sample thickness in cryo-EM[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(49): e2108884118. doi: 10.1073/pnas.2108884118 [3] DEROSIER D J. Where in the cell is my protein?[J]. Quarterly Reviews of Biophysics, 2021, 54: e9. doi: 10.1017/S003358352100007X [4] BISSON C, HECKSEL C W, GILCHRIST J B, et al. . Preparing lamellae from vitreous biological samples using a dual-beam scanning electron microscope for cryo-electron tomography: 1940-087X[R]. Menlo Park: SLAC National Accelerator Lab. , 2021. [5] GORELICK S, BUCKLEY G, GERVINSKAS G, et al. PIE-scope, integrated cryo-correlative light and FIB/SEM microscopy[J]. eLife, 2019, 8: e45919. doi: 10.7554/eLife.45919 [6] ARNOLD J, MAHAMID J, LUCIC V, et al. Site-specific cryo-focused ion beam sample preparation guided by 3D correlative microscopy[J]. Biophysical Journal, 2016, 110(4): 860-869. doi: 10.1016/j.bpj.2015.10.053 [7] MARION J, LE BARS R, SATIAT-JEUNEMAITRE B, et al. Optimizing CLEM protocols for plants cells: GMA embedding and cryosections as alternatives for preservation of GFP fluorescence in Arabidopsis roots[J]. Journal of Structural Biology, 2017, 198(3): 196-202. doi: 10.1016/j.jsb.2017.03.008 [8] TIAN X H, DE PACE C, RUIZ‐PEREZ L, et al. A cyclometalated iridium (III) complex as a microtubule probe for correlative super‐resolution fluorescence and electron microscopy[J]. Advanced Materials, 2020, 32(39): 2003901. doi: 10.1002/adma.202003901 [9] TUIJTEL M W, KOSTER A J, JAKOBS S, et al. Correlative cryo super-resolution light and electron microscopy on mammalian cells using fluorescent proteins[J]. Scientific Reports, 2019, 9(1): 1369. doi: 10.1038/s41598-018-37728-8 [10] KLEIN S, WIMMER B H, WINTER S L, et al. Post-correlation on-lamella cryo-CLEM reveals the membrane architecture of lamellar bodies[J]. Communications Biology, 2021, 4(1): 137. doi: 10.1038/s42003-020-01567-z [11] CARTER S D, MAGESWARAN S K, FARINO Z J, et al. Distinguishing signal from autofluorescence in cryogenic correlated light and electron microscopy of mammalian cells[J]. Journal of Structural Biology, 2018, 201(1): 15-25. doi: 10.1016/j.jsb.2017.10.009 [12] BHARAT T A M, HOFFMANN P C, KUKULSKI W. Correlative microscopy of vitreous sections provides insights into BAR-domain organization in situ[J]. Structure, 2018, 26(6): 879-886.e3. doi: 10.1016/j.str.2018.03.015 [13] WILFLING F, LEE C W, ERDMANN P S, et al. A selective autophagy pathway for phase-separated endocytic protein deposits[J]. Molecular Cell, 2020, 80(5): 764-778.e7. doi: 10.1016/j.molcel.2020.10.030 [14] ZHENG ZH H, LAURITZEN J S, PERLMAN E, et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster[J]. Cell, 2018, 174(3): 730-743.e22. doi: 10.1016/j.cell.2018.06.019 [15] LIU Y T, TAO CH L, ZHANG X K, et al. Mesophasic organization of GABAA receptors in hippocampal inhibitory synapses[J]. Nature Neuroscience, 2020, 23(12): 1589-1596. doi: 10.1038/s41593-020-00729-w [16] TURK M, BAUMEISTER W. The promise and the challenges of cryo‐electron tomography[J]. FEBS Letters, 2020, 594(20): 3243-3261. doi: 10.1002/1873-3468.13948 [17] DE WINTER D A M, HSIEH C, MARKO M, et al. Cryo‐FIB preparation of whole cells and tissue for cryo‐TEM: use of high‐pressure frozen specimens in tubes and planchets[J]. Journal of Microscopy, 2021, 281(2): 125-137. doi: 10.1111/jmi.12943 [18] CHANG I Y, RAHMAN M, HARNED A, et al. Cryo-fluorescence microscopy of high-pressure frozen C. elegans enables correlative FIB-SEM imaging of targeted embryonic stages in the intact worm[J]. Methods in Cell Biology, 2021, 162: 223-252. [19] Sartori A, Gatz R, Beck F, et al. Correlative microscopy: Bridging the gap between fluorescence light microscopy and cryo-electron tomography[J]. Journal of Structural Biology, 2007, 160(2): 135-145. [20] Schwartz CL, Sarbash VI, Ataullakhanov FI, et al. Cryo‐fluorescence microscopy facilitates correlations between light and cryo-electron microscopy and reduces the rate of photobleaching[J]. Journal of microscopy, 2007, 227(2): 98-109. [21] Schorb M, Briggs JAG. Correlated cryo-fluorescence and cryo-electron microscopy with high spatial precision and improved sensitivity[J]. Ultramicroscopy, 2014, 143(Supplement C): 24-32. [22] LI W X, STEIN S C, GREGOR I, et al. Ultra-stable and versatile widefield cryo-fluorescence microscope for single-molecule localization with sub-nanometer accuracy[J]. Optics Express, 2015, 23(3): 3770-3783. doi: 10.1364/OE.23.003770 [23] XU X J, XUE Y H, TIAN B Y, et al. Ultra-stable super-resolution fluorescence cryo-microscopy for correlative light and electron cryo-microscopy[J]. Science China Life Sciences, 2018, 61(11): 1312-1319. doi: 10.1007/s11427-018-9380-3 [24] HUSSELS M, KONRAD A, BRECHT M. Confocal sample-scanning microscope for single-molecule spectroscopy and microscopy with fast sample exchange at cryogenic temperatures[J]. Review of Scientific Instruments, 2012, 83(12): 123706. doi: 10.1063/1.4769996 [25] KUBA J, MITCHELS J, HOVORKA M, et al. Advanced cryo-tomography workflow developments–correlative microscopy, milling automation and cryo-lift-out[J]. Journal of Microscopy, 2021, 281(2): 112-124. doi: 10.1111/jmi.12939 [26] JEONG D, KIM D. Recent developments in correlative super-resolution fluorescence microscopy and electron microscopy[J]. Molecules and Cells, 2022, 45(1): 41-50. doi: 10.14348/molcells.2021.5011 [27] LE GROS M A, MCDERMOTT G, UCHIDA M, et al. High-aperture cryogenic light microscopy[J]. Journal of Microscopy, 2009, 235(1): 1-8. doi: 10.1111/j.1365-2818.2009.03184.x [28] GISKE A. CryoSTED microscopy: a new spectroscopic approach for improving the resolution of STED microscopy using low temperature[D]. Heidelberg: Universität Heidelberg, 2007. [29] WURM C A, SCHWARZ H, JANS D C, et al. Correlative STED super-resolution light and electron microscopy on resin sections[J]. Journal of Physics D:Applied Physics, 2019, 52(37): 374003. doi: 10.1088/1361-6463/ab2b31 [30] PRABHAKAR N, PEURLA M, KOHO S, et al. STED‐TEM correlative microscopy leveraging nanodiamonds as intracellular dual‐contrast markers[J]. Small, 2018, 14(5): 1701807. doi: 10.1002/smll.201701807 [31] ANDRIAN T, DELCANALE P, PUJALS S, et al. Correlating super-resolution microscopy and transmission electron microscopy reveals multiparametric heterogeneity in nanoparticles[J]. Nano Letters, 2021, 21(12): 5360-5368. doi: 10.1021/acs.nanolett.1c01666 [32] GU L SH, LI Y Y, ZHANG SH W, et al. Molecular resolution imaging by repetitive optical selective exposure[J]. Nature Methods, 2019, 16(11): 1114-1118. doi: 10.1038/s41592-019-0544-2 [33] GU L SH, LI Y Y, ZHANG SH W, et al. Molecular-scale axial localization by repetitive optical selective exposure[J]. Nature Methods, 2021, 18(4): 369-373. doi: 10.1038/s41592-021-01099-2 [34] 周文超, 李政昊, 武杰. 单分子生物检测方法及应用研究进展[J]. 中国光学(中英文),2022,15(5):878-894.ZHOU W CH, LI ZH H, WU J. Research progress of single molecule biological detection methods and applications[J]. Chinese Optics, 2022, 15(5): 878-894. (in Chinese) [35] WOLFF G, HAGEN C, GRÜNEWALD K, et al. Towards correlative super-resolution fluorescence and electron cryo-microscopy[J]. Biology of the Cell, 2016, 108(9): 245-258. doi: 10.1111/boc.201600008 [36] ROBICHAUX M A, POTTER V L, ZHANG ZH X, et al. Defining the layers of a sensory cilium with STORM and cryoelectron nanoscopy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(47): 23562-23572. doi: 10.1073/pnas.1902003116 [37] MOSER F, PRAŽÁK V, MORDHORST V, et al. Cryo-SOFI enabling low-dose super-resolution correlative light and electron cryo-microscopy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(11): 4804-4809. doi: 10.1073/pnas.1810690116 [38] HOFFMAN D P, SHTENGEL G, XU C S, et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells[J]. Science, 2020, 367(6475): eaaz5357. doi: 10.1126/science.aaz5357 [39] LIU B, XUE Y H, ZHAO W, et al. Three-dimensional super-resolution protein localization correlated with vitrified cellular context[J]. Scientific Reports, 2015, 5: 13017. doi: 10.1038/srep13017 [40] PHILLIPS M A, HARKIOLAKI M, PINTO D M S, et al. CryoSIM: super-resolution 3D structured illumination cryogenic fluorescence microscopy for correlated ultrastructural imaging[J]. Optica, 2020, 7(7): 802-812. doi: 10.1364/OPTICA.393203 [41] SCHERTEL A, KIRMSE R, HUMMEL E, et al.. Imaging of vitrified biological specimens by confocal cryo fluorescence microscopy and cryo FIB/SEM tomography[C]. European Microscopy Congress 2016. 2016. [42] ZACHS T, SCHERTEL A, MEDEIROS J, et al. Fully automated, sequential focused ion beam milling for cryo-electron tomography[J]. eLife, 2020, 9: e52286. doi: 10.7554/eLife.52286 [43] WU G H, MITCHELL P G, GALAZ-MONTOYA J G, et al. Multi-scale 3D cryo-correlative microscopy for vitrified cells[J]. Structure, 2020, 28(11): 1231-1237.e3. doi: 10.1016/j.str.2020.07.017 [44] LI SH G, JI G, SHI Y, et al. High-vacuum optical platform for cryo-CLEM (HOPE): a new solution for non-integrated multiscale correlative light and electron microscopy[J]. Journal of Structural Biology, 2018, 201(1): 63-75. doi: 10.1016/j.jsb.2017.11.002 [45] FAAS F G A, BÁRCENA M, AGRONSKAIA A V, et al. Localization of fluorescently labeled structures in frozen-hydrated samples using integrated light electron microscopy[J]. Journal of Structural Biology, 2013, 181(3): 283-290. doi: 10.1016/j.jsb.2012.12.004 [46] Optical path of the METEOR system. 2021. [EP/OL]. http://www.delmic.com/en/products/cryo-solutions/meteor [47] SMEETS M, BIEBER A, CAPITANIO C, et al. Integrated cryo-correlative microscopy for targeted structural investigation in situ[J]. Microscopy Today, 2021, 29(6): 20-25. doi: 10.1017/S1551929521001280 [48] BIEBER A, CAPITANIO C, SCHIØTZ O, et al. Precise 3D-correlative FIB-milling of biological samples using METEOR, an integrated cryo-CLEM imaging system[J]. Microscopy and Microanalysis, 2021, 27(S1): 3230-3232. doi: 10.1017/S1431927621011132 [49] SCHWARTZ C L, SARBASH V I, ATAULLAKHANOV F I, et al. Cryo-fluorescence microscopy facilitates correlations between light and cryo-electron microscopy and reduces the rate of photobleaching[J]. Journal of Microscopy, 2007, 227(2): 98-109. doi: 10.1111/j.1365-2818.2007.01794.x [50] DAHLBERG P D, MOERNER W E. Cryogenic super-resolution fluorescence and electron microscopy correlated at the nanoscale[J]. Annual Review of Physical Chemistry, 2021, 72: 253-278. doi: 10.1146/annurev-physchem-090319-051546 -





 下载:
下载: