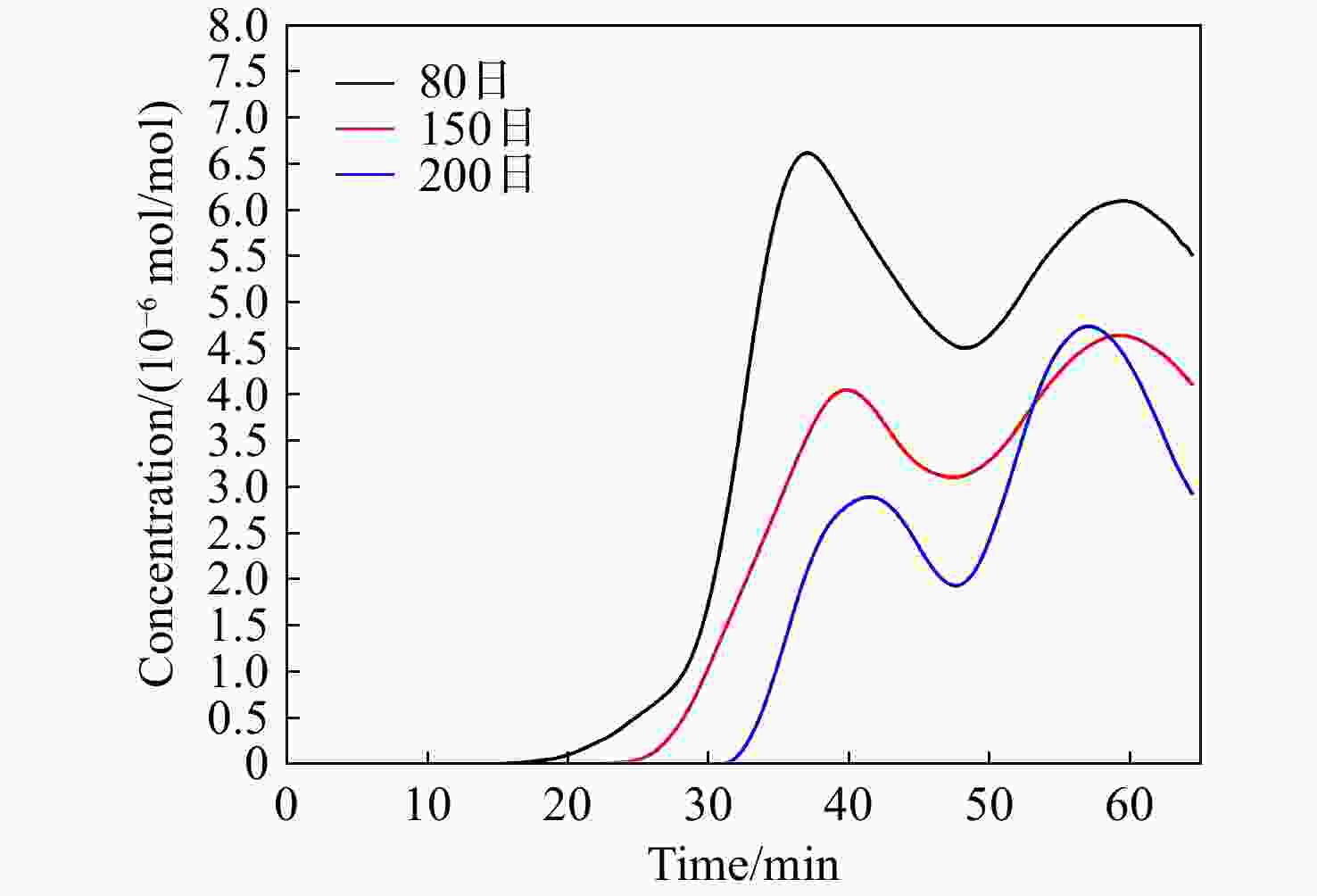
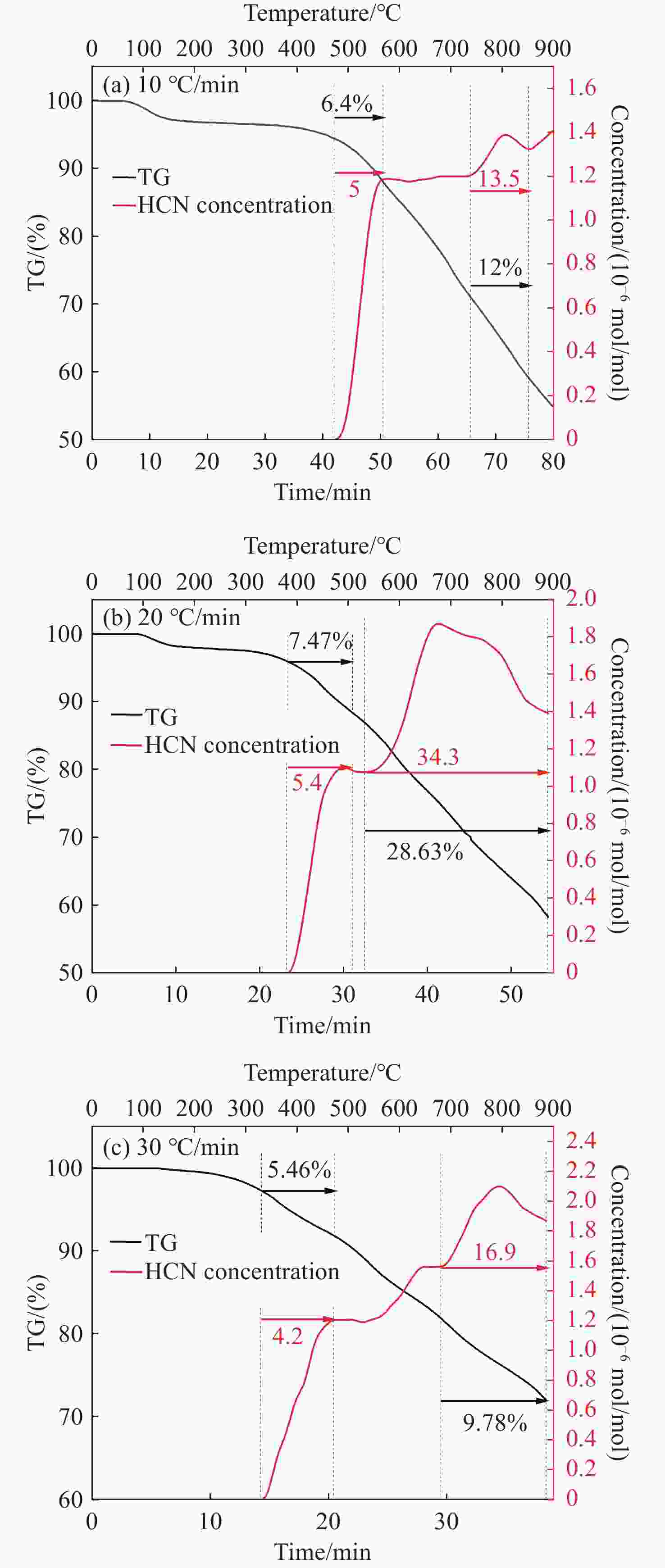
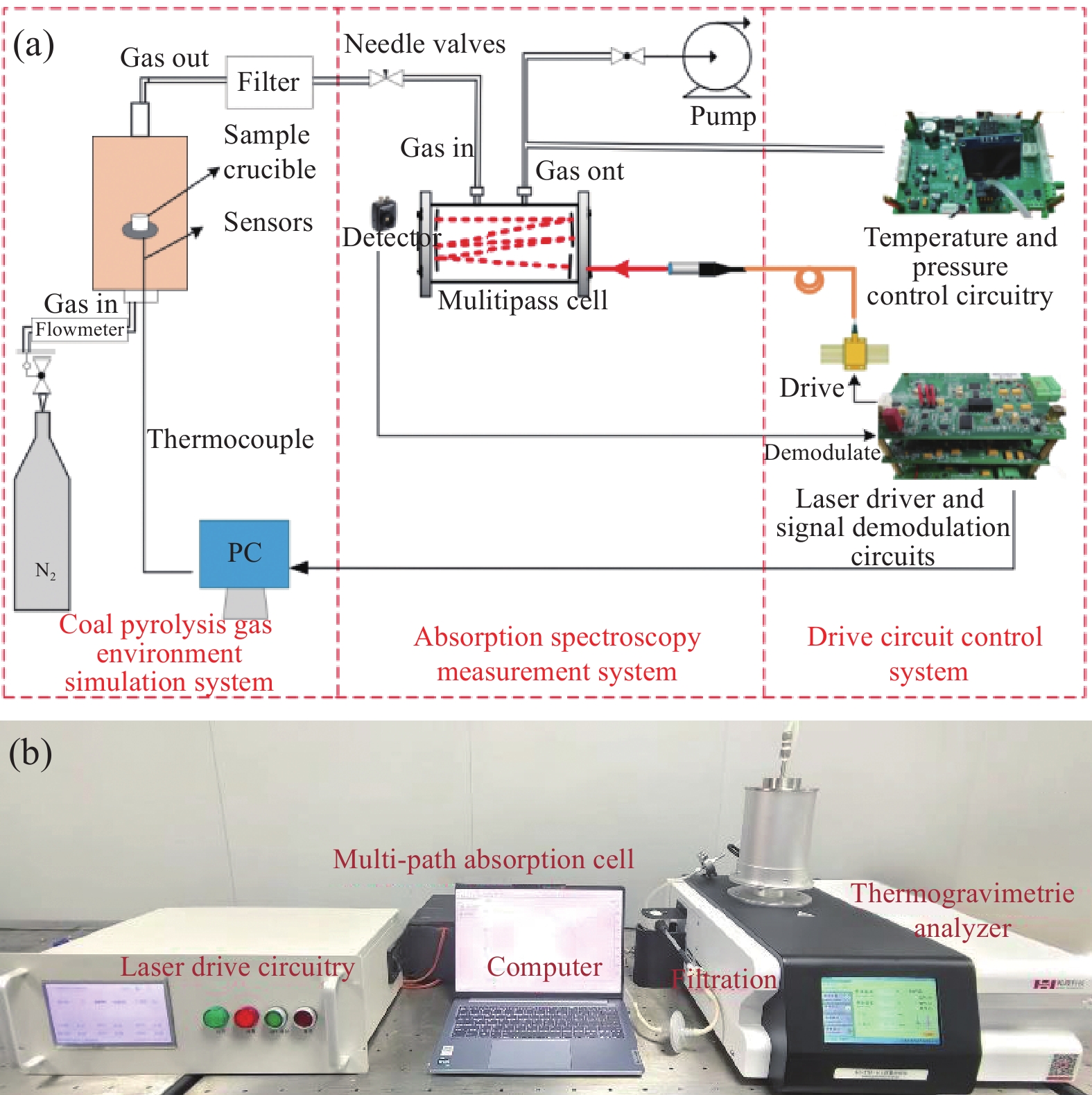
-
摘要:
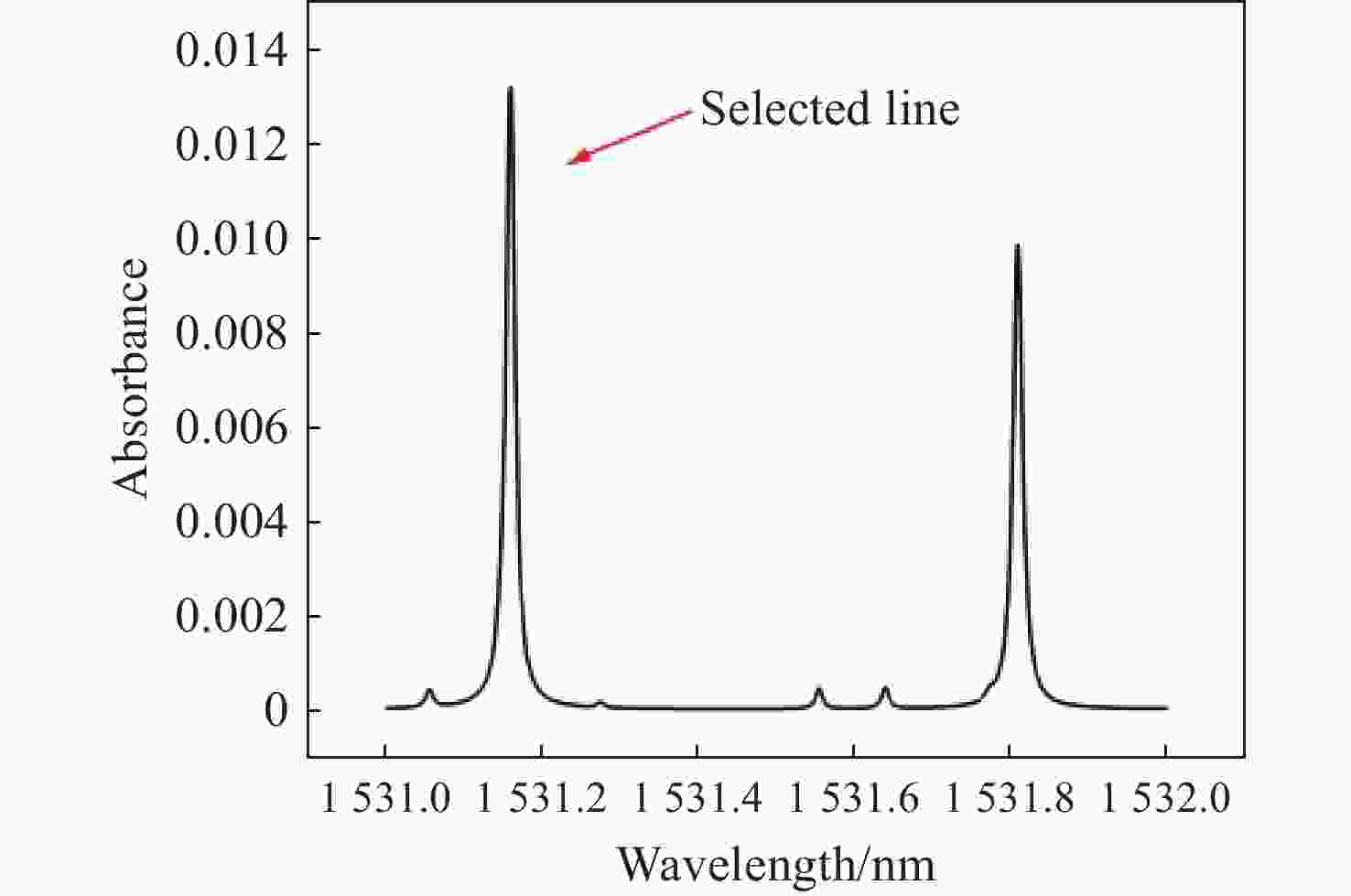
为构建一套基于热重-可调谐半导体激光吸收光谱(TG-TDLAS)技术的煤热解HCN气体浓度检测系统,并结合波长调制技术进一步提高系统的稳定性和灵敏度,本文利用HCN在波长
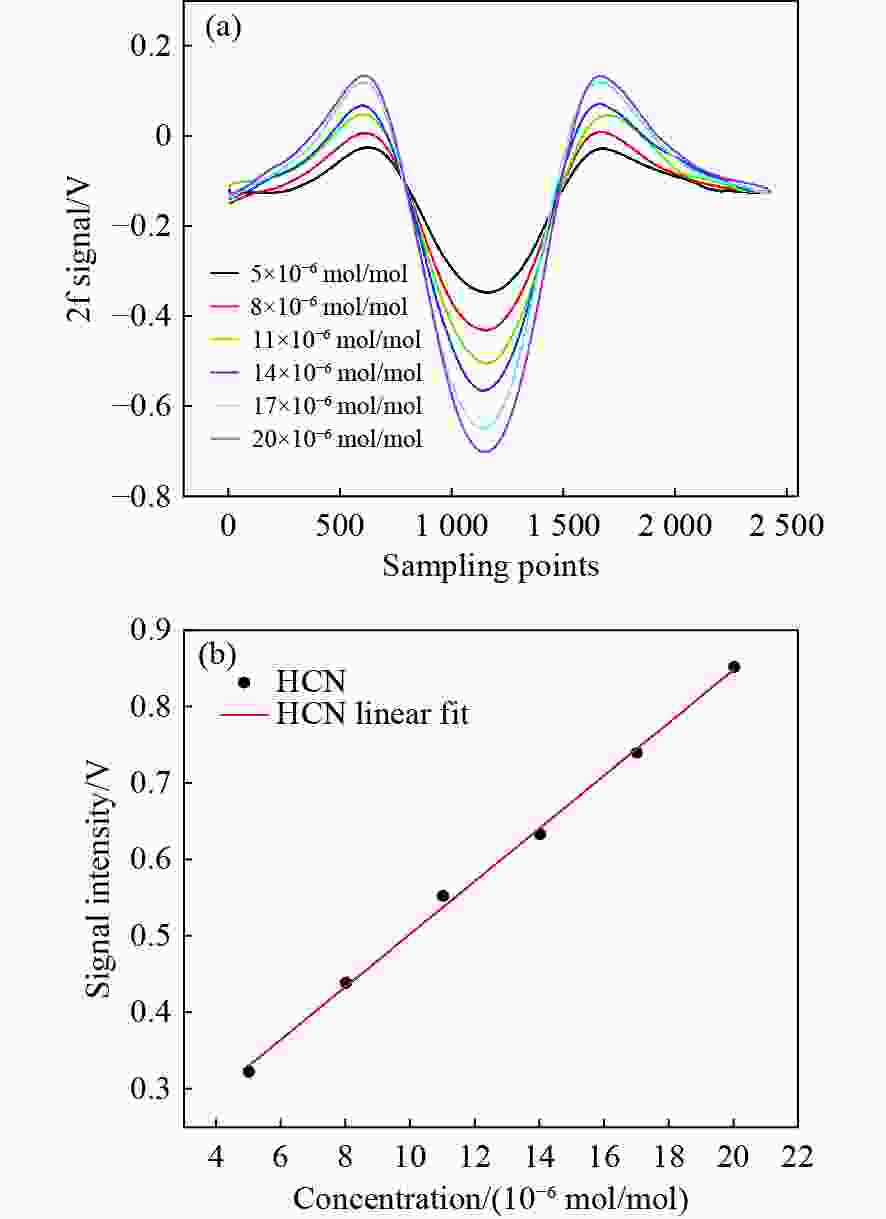
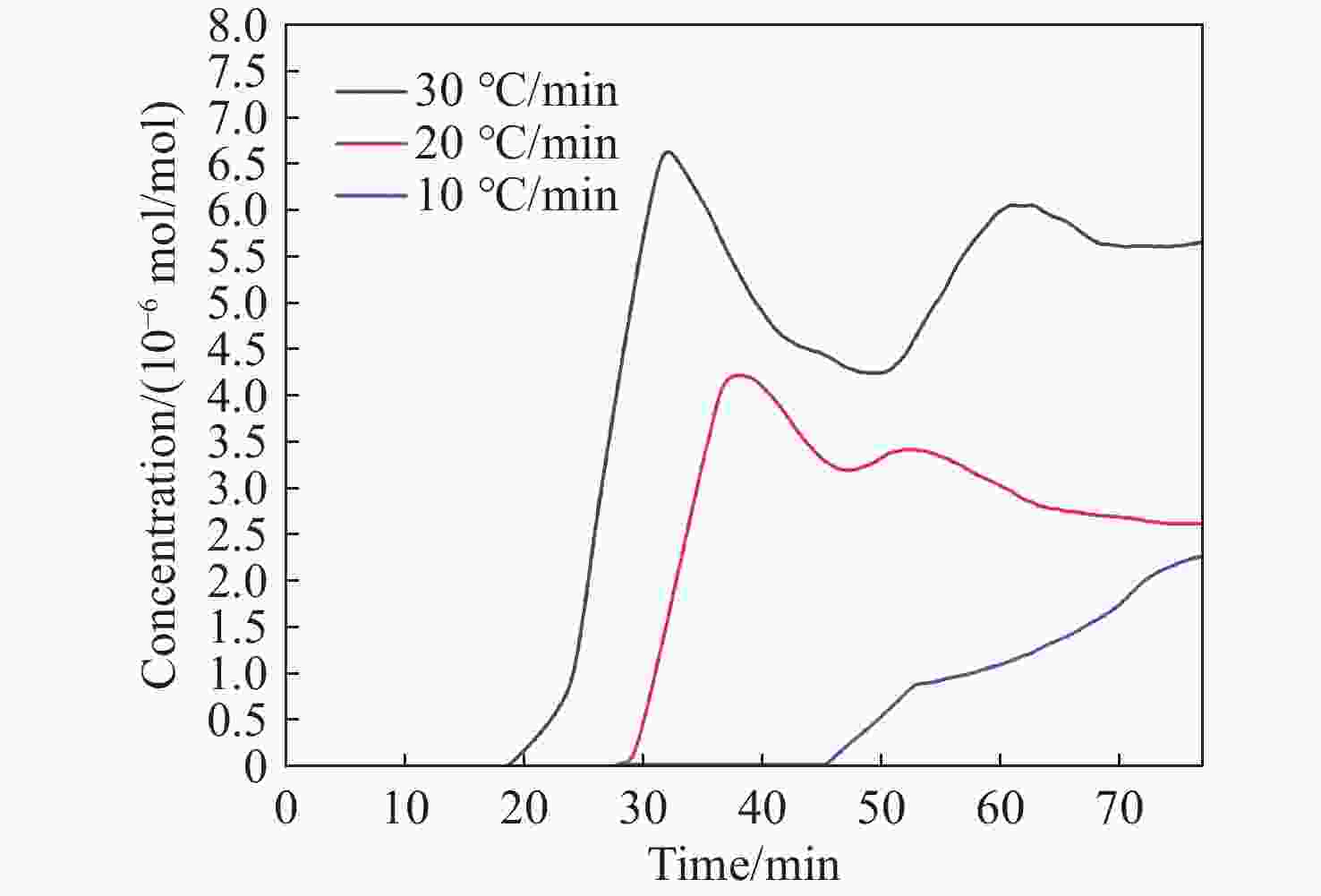
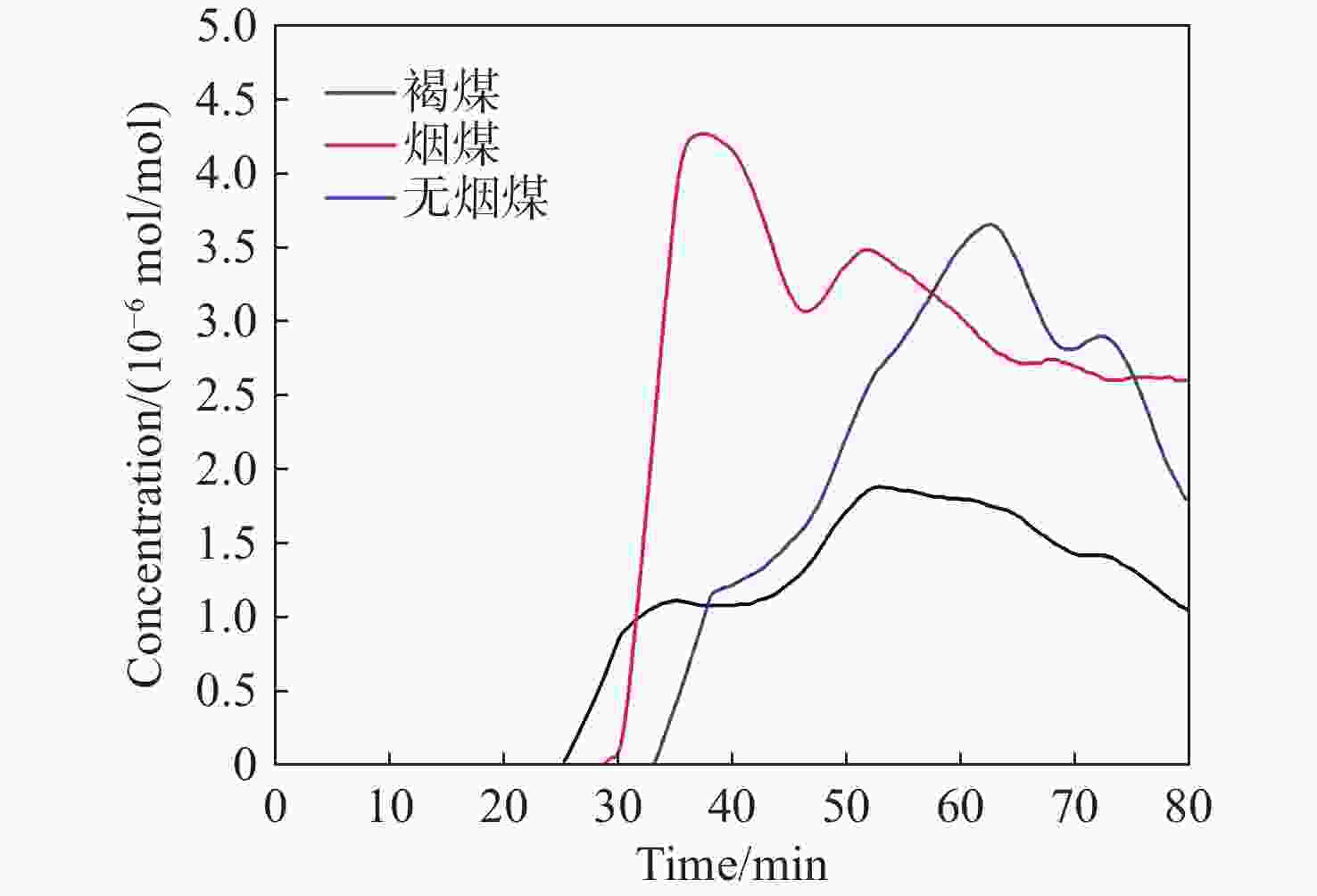
1531 nm处具有较高吸收强度且受烟气中常见气体干扰较小的特性,通过二次谐波信号处理获取HCN浓度信息。采用高精度的流量控制器,利用99%标准氮气稀释配比得到5×10−6 mol/mol到20×10−6 mol/mol的HCN,最后对测量数据进行校准。实验结果表明,HCN的线性相关系数R2 为0.9978 。基于该实验装置,深入探讨了不同煤种、升温速率和煤粒径大小对热解的影响,以及煤样失重率与HCN浓度释放量的关系。分析了3种不同煤化程度煤种的挥发分中HCN的释放特性和非等温热解动力学。通过划分热解温度阶段,建立了热解动力学模型,并计算不同煤种在不同升温速率下的活化能和频率因子。结果表明,HCN释放量与煤种煤化程度及含氮量密切相关,煤化程度越低,含氮量越高,其HCN释放量越多。在固定的热解终温下,升温速率的增加会导致HCN释放量的增多。随着煤样粒径的减小,热解反应释放HCN的时间会相对滞后,且HCN浓度有所减少。不同热解阶段HCN浓度释放量与煤样失重率之间存在不同的对应关系,热解反应越剧烈,HCN浓度释放量与煤样失重率的比重越大。本研究为进一步评估煤热解反应过程中HCN的毒性提供了重要的实验基础。Abstract:In order to construct a coal pyrolysis HCN gas concentration detection system based on thermogravimetry-tunable diode laser absorption spectroscopy (TG-TDLAS) technology, and improve the stability and sensitivity of the system by combining wavelength modulation technology. In this paper, we take advantage of the characteristics of HCN with high absorption intensity at wavelength
1531 nm and less interference by common gases in the atmosphere, the HCN concentration information is obtained by second harmonic signal processing. A high-precision flow controller is used to obtain HCN from 5×10−6 mol/mol to 20×10−6 mol/mol using a 99% standard nitrogen dilution ratio, and the measurement data is calibrated. The experimental results show that the linear correlation coefficient R2 of HCN reaches0.9978 . Then, the effects of different coal types, heating rate, and coal particle size on pyrolysis are discussed, as well as the relationship between the coal samples’ weight loss rate and the amount of HCN concentration released. The release characteristics of HCN and the nonisothermal pyrolysis kinetics in the volatile matter of three coal types with different coalification degrees are analyzed. A pyrolysis kinetic model was established by dividing the pyrolysis temperature stages, and the activation energy and frequency factors of varying coal types at different heating rates are calculated. The results show that the HCN emission is closely related to the degree of coalification and nitrogen content of coal types. The lower the degree of coalification, the higher the nitrogen content and the more HCN emitted. Under the fixed pyrolysis final temperature, an increase in the heating rate will increase the amount of HCN released. With the decrease in coal particle size, the time of HCN release from the pyrolysis reaction will be delayed, and the HCN concentration will decrease. There was a different correspondence between the release of HCN concentration and the coal samples’ weight loss rate in different pyrolysis stages. The more intense the pyrolysis reaction, the greater the proportion of HCN concentration released to the coal samples’ weight loss rate. This study provides an important experimental basis for further evaluation of the toxicity of HCN during coal pyrolysis reactions.-
Key words:
- TG-TDLAS /
- coal pyrolysis /
- wavelength modulation /
- pyrolysis kinetics
-
表 1 不同煤种的工业分析结果
Table 1. Industrial analysis results of different coal types
全硫/Std 灰分/ Ad 挥发分/Vdaf 山西褐煤 1 26 33 山东烟煤 1.95 9.59 31.32 山东无烟煤 3.85 13.1 10.19 表 2 不同煤种在不同升温速率下的热解动力学参数
Table 2. Kinetic parameters of pyrolysis of different coal types at different heating rates
煤种 β, °C/min T, °C E,kJ/mol A, min−1 R2 褐煤 10 421~654 14.875 12.018 0.932 654~899 29.092 109.600 0.933 20 409~632 9.667 5.898 0.975 632~880 25.396 141.629 0.968 30 453~647 22.932 107.295 0.931 647~894 30.971 458.678 0.951 烟煤 10 420~640 27.519 133.482 0.990 640~870 31.750 244.656 0.965 20 443~661 22.617 103.486 0.975 661~890 27.552 182.316 0.942 30 460~643 20.100 114.484 0.933 643~890 27.061 369.150 0.941 无烟煤 10 475~708 44.614 1163.052 0.959 708~890 43.431 653.392 0.950 20 481~648 45.240 2450.536 0.942 650~890 37.665 670.213 0.949 30 480~650 42.587 2383.327 0.927 650~890 37.556 1024.036 0.931 -
[1] WANG Z H, ZHANG J Y, ZHAO Y C, et al. Relationship between nitrogenous species in coals and volatile nitrogen-containing yields during pyrolysis[J]. Asia-Pacific Journal of Chemical Engineering, 2012, 7(1): 124-130. doi: 10.1002/apj.501 [2] YUAN SH, ZHOU ZH J, LI J, et al. HCN and NH3 (NO x precursors) released under rapid pyrolysis of biomass/coal blends[J]. Journal of Analytical and Applied Pyrolysis, 2011, 92(2): 463-469. doi: 10.1016/j.jaap.2011.08.010 [3] ZHANG J L, CHEN W S, YANG H M, et al. Formation of NH3 during temperature-programmed and isotherm pyrolysis of different rank coals[J]. Asian Journal of Chemistry, 2013, 25(13): 7571-7574. doi: 10.14233/ajchem.2013.15261 [4] HE Q, CHENG CH, ZHANG X SH, et al. Insight into structural evolution and detailed non-isothermal kinetic analysis for coal pyrolysis[J]. Energy, 2022, 244: 123101. doi: 10.1016/j.energy.2022.123101 [5] WU Y N, TAO SH, WANG ZH H, et al. Effect of pyrolysis atmospheres on gaseous products evolution of coal pyrolysis at high temperature[J]. Fuel, 2024, 366: 131336. doi: 10.1016/j.fuel.2024.131336 [6] 张莹, 赵浩成, 李挺, 等. 不同热解升温速率下烟煤热解焦结构特性及其气化反应性的研究[J]. 广东化工,2023,50(6):35-38,92. doi: 10.3969/j.issn.1007-1865.2023.06.012ZHANG Y, ZHAO H CH, LI T, et al. Study on structure and gasification reactivity of char from different pyrolysis heating rates[J]. Guangdong Chemical Industry, 2023, 50(6): 35-38,92. (in Chinese). doi: 10.3969/j.issn.1007-1865.2023.06.012 [7] GHANEKAR S, HORN G P, KESLER R M, et al. Quantification of elevated hydrogen cyanide (HCN) concentration typical in a residential fire environment using mid-IR tunable diode laser[J]. Applied Spectroscopy, 2023, 77(4): 382-392. doi: 10.1177/00037028231152498 [8] SUN L SH, SHI J M, XIANG J, et al. Study on the release characteristics of HCN and NH3 during coal gasification[J]. Asia-Pacific Journal of Chemical Engineering, 2010, 5(3): 403-407. doi: 10.1002/apj.276 [9] YUAN SH, ZHOU ZH J, LI J, et al. Nitrogen conversion during rapid pyrolysis of coal and petroleum coke in a high-frequency furnace[J]. Applied Energy, 2012, 92: 854-859. doi: 10.1016/j.apenergy.2011.08.042 [10] LIU J X, JIANG X M, SHEN J, et al. Pyrolysis of superfine pulverized coal. Part 3. Mechanisms of nitrogen-containing species formation[J]. Energy Conversion and Management, 2015, 94: 130-138. doi: 10.1016/j.enconman.2014.12.096 [11] SONG H J, LIU G R, ZHANG J ZH, et al. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Processing Technology, 2017, 156: 454-460. doi: 10.1016/j.fuproc.2016.10.008 [12] XU M X, LI SH Y, WU Y H, et al. Effects of CO2 on the fuel nitrogen conversion during coal rapid pyrolysis[J]. Fuel, 2016, 184: 430-439. doi: 10.1016/j.fuel.2016.06.130 [13] MI Q Y, LI B, LI Y F, et al. Kinetic analysis of pyrolysis reaction of hydrogen-containing low rank coals based on thermogravimetric method[J]. Processes, 2023, 11(3): 706. doi: 10.3390/pr11030706 [14] 段政, 孟星星, 李凯亮, 等. 煤热解中痕量乙烯在线激光吸收光谱检测[J]. 光学 精密工程,2024,32(5):670-677. doi: 10.37188/OPE.20243205.0670DUAN ZH, MENG X X, LI K L, et al. Online laser absorption spectroscopy detection of trace ethylene in coal pyrolysis[J]. Optics and Precision Engineering, 2024, 32(5): 670-677. (in Chinese). doi: 10.37188/OPE.20243205.0670 [15] 杨舒涵, 乔顺达, 林殿阳, 等. 基于可调谐半导体激光吸收光谱的氧气浓度高灵敏度检测研究[J]. 中国光学(中英文),2023,16(1):151-157. doi: 10.37188/CO.2022-0029YANG SH H, QIAO SH D, LIN D Y, et al. Research on highly sensitive detection of oxygen concentrations based on tunable diode laser absorption spectroscopy[J]. Chinese Optics, 2023, 16(1): 151-157. (in Chinese). doi: 10.37188/CO.2022-0029 [16] 裴梓伊, 胡朋兵, 潘孙强, 等. TDLAS气体激光遥测高灵敏光电探测电路设计[J]. 中国光学(中英文),2024,17(1):198-208. doi: 10.37188/CO.2023-0107PEI Z Y, HU P B, PAN S Q, et al. Design of a highly sensitive photoelectric detection circuit for TDLAS gas laser telemetry[J]. Chinese Optics, 2024, 17(1): 198-208. (in Chinese). doi: 10.37188/CO.2023-0107 [17] 李文军, 陈姗姗, 陈艳鹏, 等. 基于热重的煤热解反应动力学试验研究[J]. 中国煤炭,2020,46(3):84-89. doi: 10.3969/j.issn.1006-530X.2020.03.017LI W J, CHEN SH SH, CHEN Y P, et al. Experimental study on coal pyrolysis kinetics based on thermogravimetry analysis[J]. China Coal, 2020, 46(3): 84-89. (in Chinese). doi: 10.3969/j.issn.1006-530X.2020.03.017 [18] 彭扬凡, 陈姗姗, 孙粉锦, 等. 基于热重法的大颗粒煤热解反应动力学[J]. 洁净煤技术,2021,27(6):128-133.PENG Y F, CHEN SH SH, SUN F J, et al. Investigation on the kinetics of pyrolysis reaction of large coal particles based on TGA[J]. Clean Coal Technology, 2021, 27(6): 128-133. (in Chinese). [19] LI CH ZH, TAN L L. Formation of NO x and SO x precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel, 2000, 79(15): 1899-1906. doi: 10.1016/S0016-2361(00)00008-9 [20] 张肖阳, 周滨选, 安东海, 等. 升温速率对准东褐煤热解特性及煤焦孔隙结构的影响[J]. 煤炭学报,2019,44(2):604-610.ZHANG X Y, ZHOU B X, AN D H, et al. Effect of heating rate on pyrolysis characteristics and char structure of Zhundong lignite coal[J]. Journal of China Coal Society, 2019, 44(2): 604-610. (in Chinese). [21] TIAN B, QIAO Y Y, TIAN Y Y, et al. Investigation on the effect of particle size and heating rate on pyrolysis characteristics of a bituminous coal by TG–FTIR[J]. Journal of Analytical and Applied Pyrolysis, 2016, 121: 376-386. doi: 10.1016/j.jaap.2016.08.020 [22] WANG H W, DU W ZH, XI Y, et al. Non-isothermal thermogravimetric analysis study on the pyrolysis reaction kinetics of bituminous coal[J]. Chemical Engineering & Technology, 2022, 45(6): 1048-1057. -





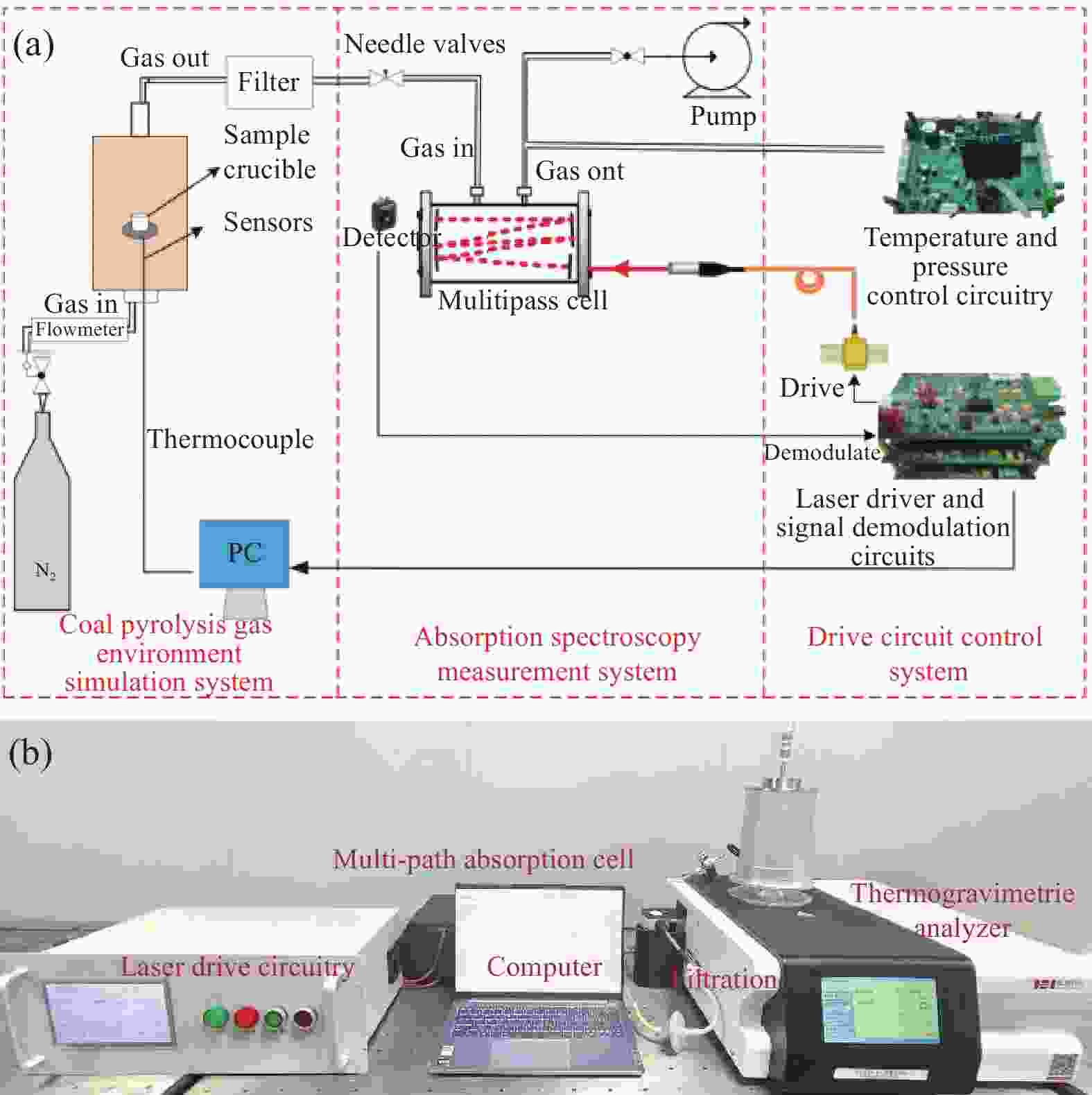
 下载:
下载: