Luminescene properties and various mechanisms of rare earth ions in the nanocrystals
-
摘要: 近年来, 纳米晶体中稀土离子发光性质的研究越来越受到人们的广泛关注, 这是因为纳米稀土发光材料在发光、高清显示、光电子纳米器件、生物荧光标记、激光和闪烁体等众多领域有着重要的应用前景。本项目采用软化学合成方法如水热法、溶胶-凝胶法等, 通过合成工艺的调控, 设计并合成出一系列不同颗粒尺寸、分散均匀、形貌可控的稀土离子掺杂氧化物(氟化物)微/纳米晶体, 利用激发、发射、漫反射以及高分辨激光光谱等光谱分析手段对其发光性质进行研究, 弄清影响发光行为的本质原因。同时, 结合光谱实验数据, 利用密度泛函理论和复杂晶体化学键介电理论方法进行理论计算, 成功解释了光谱变化规律和不同稀土离子间能量传递机理, 为相关稀土光谱研究奠定了理论和实验基础。Abstract: In recent years, the luminescence properties of the rare earth ions-doped nanocrystals become more and more popular because the rare earth luminescent nanocrystals have important applications in photoluminescence, high-definition display, optoelectronic nano-devices, biological labeling, laser and scintillator etc.. In this project, a series of different particle size, dispersion, controllable morphology rare earth ions-doped oxide(fluoride) micro-/nano-crystals were synthesized by soft chemistry synthesis method such as hydrothermal, sol-gel method et al.. The luminescence properties were investigated by excitation spectra, emission spectra, diffuse reflectance spectra and high-resolution laser spectra in order to observe the changes of luminescent phenomenon with different size and chemical bond. The essential reason of the impact of luminescence behavior was also investigated by summary and classification. At the same time, theoretical calculations of optical properties were performed by using density functional theory and complex crystal dielectric theory of chemical bond method with the combination of spectral experimental data, which can successfully explain the spectral shift rule and energy transfer mechanism between the different rare earth ions. The results can lay the theoretical and experimental basis for the other rare earth spectroscopy.
-
Key words:
- micro-/nano-crystals /
- rare earth ions /
- charge transfer band /
- energy transfer
-
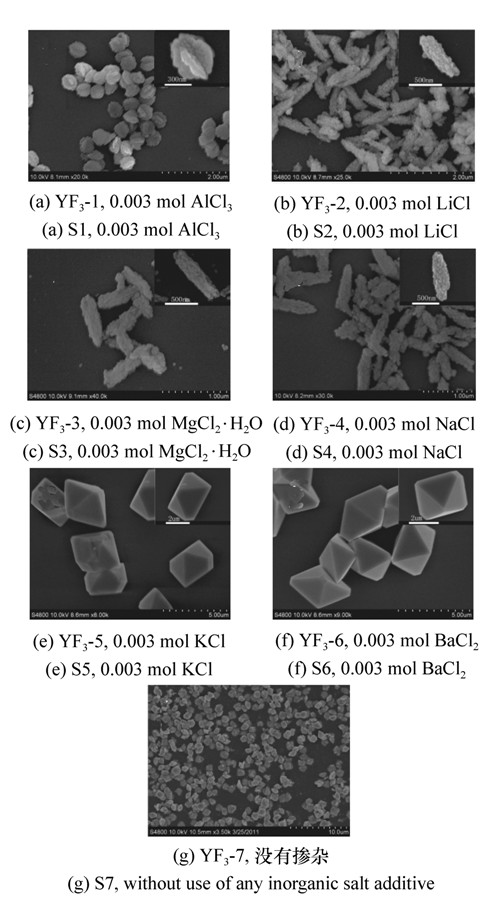
图 2 掺杂不同无机盐YF3∶Eu3+的激发光谱和发射光谱:(S1)YF3-1,0.003 mol AlCl3; (S2)YF3-2,0.003 mol LiCl; (S3)YF3-3,0.003 mol MgCl2·6H2O; (S4)YF3-4,0.003 mol NaCl; (S5)YF3-5,0.003 mol KCl; (S6)YF3-6,0.003 mol BaCl2
Figure 2. PLE and PL emission spectra of YF3∶Eu3+ with different doped inorganic salts: (a)S1,0.003 mol AlCl3; (b)S2,0.003 mol LiCl; (c)S3,0.003 mol MgCl2·6H2O; (d)S4,0.003 mol NaCl; (e)S5,0.003 mol KCl; (f)S6,0.003 mol BaCl2.
表 1 LaAlO3 和GdAlO3的平均化学键参数及电荷迁移能理论值
Table 1. Average chemical bond parameters and theoretical values of charge tranfer energy of LaAlO3 and GdAlO3
样品 化学键 键长 共价性 平均能隙/eV 电荷迁移能/eV LaAlO3 La—O 2.664 5 0.121 9 10.025 2 4.25 GdAlO3 Gd—O 2.455 8 0.088 5 14.741 2 5.06 -
[1] [2] [3] [4] [5] [6] [7] [8] -






 下载:
下载: