-
摘要: 纳米载体一直是肿瘤精准治疗的重要研究领域。其中以细胞膜伪装的纳米药物载体作为一种新颖的药物载体平台,在近年来已成为药物传递领域的研究热点。本文综述了不同种类细胞膜伪装的纳米载体应用于光热治疗的最新进展。将细胞膜与纳米材料结合起来,可进一步推进纳米载体的研究,这将对相关领域的发展产生重要影响。Abstract: Nanocarriers have always been an important research area of the accurate tumor therapy. As a novel drug carrier platform, cell membrane-camouflaged nano drug carriers have become a research hot area in the drug delivery field in recent years. This paper reviews the latest advances in the application for photothermal therapy of different cell membrane-camouflaged nano-carriers. Combining cell membranes with nanomaterials can further improve the research of nanocarriers and have important implications for the development of related fields.
-
Key words:
- nanocarrier /
- cell membrane /
- camouflage /
- erythrocyte /
- stem cell /
- photothermal therapy
-
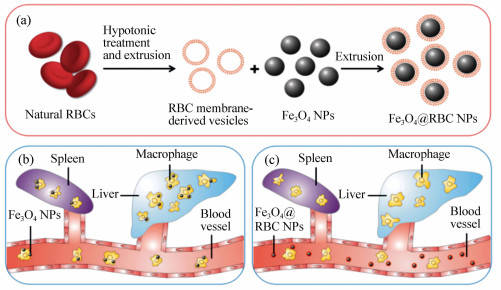
图 1 制备和功能化红细胞伪装在Fe3O4纳米颗粒表面(Fe3O4@RBC NPs)。(a)红细胞膜伪装在Fe3O4纳米颗粒制备过程。(b)未伪装的Fe3O4纳米颗粒被网状内皮系统吞噬。(c)Fe3O4@RBC NPs能网状内皮系统吞噬逃逸
Figure 1. Schematic of preparation and functionality of erythrocyte membrane-camouflaged Fe3O4 nanoparticles(Fe3O4@RBC NPs). (a)Preparation process of surface camouflage of Fe3O4 NPs with RBC membranes; (b)uncoated Fe3O4 NPs are phagocytized by the reticuloendothelial system; (c)Fe3O4@RBC NPs can escape the RES uptake
图 2 微流控电穿孔促进合成RBC-MNs用于提高成像介导的癌症治疗。(a)使用微流控电穿孔合成RBC-MNs。(b)RBC-MNs从微流控芯片收集,经过血液循环后富集在肿瘤部位。(c)仿生RBC-MNs进一步用于提高肿瘤部位MRI核磁共振成像和PTT治疗
Figure 2. Microfluidic electroporation-facilitated synthesis of RBC-MNs for enhanced imaging-guided cancer therapy. (a)Microfluidic electroporation facilitates the synthesis of RBC-MNs; (b)subsequently, the RBC-MNs, which are collected from the microfluidic chip, enrich in the tumor site after the blood circulation; (c)biomimetic RBC-MNs are further used for enhanced in vivo tumor MRI and PTT
图 3 血小板仿生纳米颗粒用来增强癌症成像和治疗。(A)血小板从小鼠血液中分离。(B,C)血小板膜囊泡随着膜蛋白质被收集并进一步包裹在Fe3O4纳米颗粒表面。(D)制备的PLT-MNs静脉注射到小鼠体内。(E,F)经系统循环后,PLT-MNs通过EPR效应富集在肿瘤部位。(G)由于PLTs的肿瘤靶向特性,PLT-MNs能靶向到肿瘤细胞。(H,I)为了利用MNs磁特性和光学吸收性质,我们将仿生PLT-MNs用于MRI和光热治疗
Figure 3. Platelet-mimicking magnetic nanoparticles for enhanced cancer imaging and therapy. (A)Platelets(PLTs) were separated from mice blood; (B, C)PLT membrane-derived vesicles(PLT-vesicles) along with the membrane proteins were collected from the PLTs and further coated onto Fe3O4 magnetic nanoparticles(MNs); (D)subsequently, the resulting PLT membrane-coated MNs(PLT-MNs) were intravenous (i.v.)injected back into the donor mice; (E, F)after systematic circulation, PLT-MNs enriched in the tumor site via the enhanced permeability and retention(EPR) effect; (G)attributed to the cancer targeting ability inherited from PLTs, PLT-MNs closely bonded to cancer cells; (H, I)to exploit the magnetic property and optical absorption ability of MNs, our biomimetic PLT-MNs were then used for enhanced in vivo tumor magnetic resonance imaging(MRI) and photothermal therapy(PTT)
-
[1] MITRAGOTRI S, BURKE PA, LANGER R. Overcoming the challenges in administering biopharmaceuticals:formulation and delivery strategies[J]. Nature Reviews Drug Discovery, 2014, 13(9):655-672. doi: 10.1038/nrd4363 [2] MATSUMURA Y, MAEDA H. A new concept for macromolecular therapeutics in cancer chemotherapy:mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Research, 1986, 46(12 Part 1):6387-6392. http://cn.bing.com/academic/profile?id=bf77b5dc058644475ce5c6ff405be910&encoded=0&v=paper_preview&mkt=zh-cn [3] XIE J, XU C, KOHLER N, et al.. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells[J]. Advanced Materials, 2007, 19(20):3163-3166. doi: 10.1002/adma.200701975 [4] PETROS R A, DESIMONE J M. Strategies in the design of nanoparticles for therapeutic applications[J]. Nature Reviews Drug Discovery, 2010, 9:615. doi: 10.1038/nrd2591 [5] LIU Z, ROBINSON J T, SUN X, et al.. PEGylated nanographene oxide for delivery of water-Insoluble cancer drugs[J]. Journal of the American Chemical Society, 2008, 130(33):10876-10877. doi: 10.1021/ja803688x [6] ZHANG C Y, YEH H C, KUROKI M T, et al.. Single-quantum-dot-based DNA nanosensor[J]. Nature Materials, 2005, 4(11):826-831. doi: 10.1038/nmat1508 [7] KNOP K, HOOGENBOOM R, FISCHER D, et al.. Poly(ethylene glycol) in drug delivery:pros and cons as well as potential alternatives[J]. Angewandte Chemie International Edition, 2010, 49(36):6288-6308. doi: 10.1002/anie.200902672 [8] WILHELM S, TAVARES AJ, DAI Q, et al.. Analysis of nanoparticle delivery to tumours[J]. Nature Reviews Materials, 2016, 1(5):16014. doi: 10.1038/natrevmats.2016.14 [9] WANG S, HUANG P, CHEN X. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization[J]. Advanced Materials, 2016, 28(34):7340-7364. doi: 10.1002/adma.201601498 [10] HUANG C, YANG G, HA Q, et al. Multifunctional "smart" particles engineered from live immunocytes:toward capture and release of cancer cells[J]. Advanced Materials, 2015, 27(2):310-313. doi: 10.1002/adma.v27.2 [11] TANG R, MOYANO DF, SUBRAMANI C, et al.. Rapid coating of surfaces with functionalized nanoparticles for regulation of cell behavior[J]. Advanced Materials, 2014, 26(20):3310-3314. doi: 10.1002/adma.v26.20 [12] FANG R H, JIANG Y, FANG J C, et al.. Cell membrane-derived nanomaterials for biomedical applications[J]. Biomaterials, 2017, 128(Supplement C):69-83. http://cn.bing.com/academic/profile?id=e3696cec8291b081c454f71e90dbcd26&encoded=0&v=paper_preview&mkt=zh-cn [13] SUN H P, SU J H, MENG Q S, et al.. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer[J]. Advanced Functional Materials, 2017, 27(3):1604300-n/a. doi: 10.1002/adfm.v27.3 [14] SUN H, SU J, MENG Q, et al.. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors[J]. Advanced Materials, 2016, 28(43):9581-9588. doi: 10.1002/adma.201602173 [15] GAO W, HU C-M J, FANG R H, et al.. Surface functionalization of gold nanoparticles with red blood cell membranes[J]. Advanced Materials, 2013, 25(26):3549-3553. doi: 10.1002/adma.201300638 [16] KROLL AV, FANG RH, ZHANG L. Biointerfacing and applications of cell membrane-coated nanoparticles[J]. Bioconjugate Chemistry, 2017, 28(1):23-32. doi: 10.1021/acs.bioconjchem.6b00569 [17] LI S-Y, QIU W X, CHENG H, et al.. A versatile plasma membrane engineered cell vehicle for contact-cell-enhanced photodynamic therapy[J]. Advanced Functional Materials, 2017, 27(12):1604916-n/a. doi: 10.1002/adfm.v27.12 [18] TIAN H, LUO Z, LIU L, et al.. Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance[J]. Advanced Functional Materials, 2017, 27(38):1703197-n/a. doi: 10.1002/adfm.v27.38 [19] FU Q, LV P, CHEN Z, et al.. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane[J]. Nanoscale, 2015, 7(9):4020-4030. doi: 10.1039/C4NR07027E [20] DEHAINI D, WEI X, FANG R H, et al.. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization[J]. Advanced Materials, 2017, 29(16):1606209-n/a. doi: 10.1002/adma.201606209 [21] CHEN Z, ZHAO P, LUO Z, et al.. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy[J]. ACS Nano, 2016, 10(11):10049-10057. doi: 10.1021/acsnano.6b04695 [22] LUK B T, FANG R H, HU C-M J, et al.. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors[J]. Theranostics, 2016, 6(7):1004-1011. doi: 10.7150/thno.14471 [23] SU J, SUN H, MENG Q, et al.. Long circulation red-blood-cell-mimetic nanoparticles with peptide-enhanced tumor penetration for simultaneously inhibiting growth and lung metastasis of breast cancer[J]. Advanced Functional Materials, 2016, 26(8):1243-1252. doi: 10.1002/adfm.v26.8 [24] LI S Y, CHENG H, XIE B R, et al.. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy[J]. ACS Nano, 2017, 11(7):7006-7018. doi: 10.1021/acsnano.7b02533 [25] WANG X, LI H, LIU X, et al.. Enhanced photothermal therapy of biomimetic polypyrrole nanoparticles through improving blood flow perfusion[J]. Biomaterials, 2017, 143(Supplement C):130-141. http://cn.bing.com/academic/profile?id=d8ed9365d0b05aef02359485bc53cfa7&encoded=0&v=paper_preview&mkt=zh-cn [26] REN X, ZHENG R, FANG X, et al.. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy[J]. Biomaterials, 2016, 92(Supplement C):13-24. http://cn.bing.com/academic/profile?id=3c8c41999685f2c66f7ba9e881ad8b2e&encoded=0&v=paper_preview&mkt=zh-cn [27] ZHANG Z, WANG J, NIE X, et al.. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods[J]. Journal of the American Chemical Society, 2014, 136(20):7317-7326. doi: 10.1021/ja412735p [28] MELANCON M P, ZHOU M, LI C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles[J]. Accounts of Chemical Research, 2011, 44(10SI):947-956. http://cn.bing.com/academic/profile?id=83b56c30ef5304f92c08426f0a6e5fb1&encoded=0&v=paper_preview&mkt=zh-cn [29] CHENG L, GONG H, ZHU W, et al.. PEGylated prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy[J]. Biomaterials, 2014, 35(37):9844-9852. doi: 10.1016/j.biomaterials.2014.09.004 [30] HU C-M J, ZHANG L, ARYAL S, et al.. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform[J]. Proceedings of the National Academy of Sciences, 2011, 108(27):10980-10985. doi: 10.1073/pnas.1106634108 [31] LANG R, JUN-HUA X, BO C, et al.. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake[J]. Nanotechnology, 2016, 27(8):085106. doi: 10.1088/0957-4484/27/8/085106 [32] RAO L, MENG Q F, HUANG Q, et al.. Photocatalytic degradation of cell membrane coatings for controlled drug release[J]. Advanced Healthcare Materials, 2016, 5(12):1420-1427. doi: 10.1002/adhm.201600303 [33] RAO L, CAI B, BU L L, et al.. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy[J]. ACS Nano, 2017, 11(4):3496-3505. doi: 10.1021/acsnano.7b00133 [34] RAO L, BU L L, MENG Q F, et al.. Antitumor platelet-mimicking magnetic nanoparticles[J]. Advanced Functional Materials, 2017, 27(9):1604774-n/a. doi: 10.1002/adfm.201604774 [35] HU Q, SUN W, QIAN C, et al.. Anticancer platelet-mimicking nanovehicles[J]. Advanced Materials, 2015, 27(44):7043-7050. doi: 10.1002/adma.201503323 [36] RAO L, BU L L, CAI B, et al.. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging[J]. Advanced Materials, 2016, 28(18):3460-3466. doi: 10.1002/adma.201506086 [37] GAO W, FANG R H, THAMPHIWATANA S, et al. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles[J]. Nano Letters, 2015, 15(2):1403-1409. doi: 10.1021/nl504798g [38] GAO C, LIN Z, JURADO-SÁNCHEZ B, et al.. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery[J]. Small, 2016, 12(30):4056-4062. doi: 10.1002/smll.v12.30 [39] GAO C, LIN Z, WU Z, et al.. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy[J]. ACS Applied Materials & Interfaces, 2016, 8(50):34252-34260. http://cn.bing.com/academic/profile?id=efe298a12b0b619b18a0e69a3a6370c5&encoded=0&v=paper_preview&mkt=zh-cn -






 下载:
下载: