-
摘要: 碳纳米点作为新兴的碳纳米材料,具备制备成本低、尺寸小、低毒、生物相容性高、水溶性好、易修饰、光物理性质独特等诸多优点,在生物医疗领域展现了独有的优势和应用前景。由于具有丰富的表面官能团,碳纳米点可以与靶向配体、医学影像造影剂、核酸、化学药物、光敏剂、光热转换试剂等功能性诊断治疗试剂相互作用形成复合物。目前,碳纳米点及其复合物在医学影像、基因治疗、化学药物治疗、光热、光动力治疗等生物医学诊断治疗领域的应用正在被广泛的开发和报道。这些工作对开发基于碳纳米点的医学诊断治疗试剂及其临床推进具有重要意义,为推进人类重大疾病的个体化、可视化、非入侵式、小损伤的诊断治疗提供一种新的药物体系。本文将关注应用于诊断治疗领域的碳纳米点及其复合物的设计、构建及性能研究,对已报道的基于碳纳米点的诊断治疗试剂在生物医疗领域的研究进展进行总结和讨论。Abstract: As an emerging carbon nanomaterial, carbon nanodots(CNDs) have many advantages such as low preparation cost, small size, low toxicity, high biocompatibility, good water solubility, easy modification, unique photophysical properties, and exhibit unique advantages and application prospects in the field of biomedicine. Taking advantage of the abundant surface functional groups, carbon nanodots can interact with functional theranostic agents such as targeting ligands, contrast agents in medical imaging, nucleic acids, chemical drugs, photosensitizers, and photothermal conversion reagents to form composites. Currently, bioluminescent imaging applications of carbon nanodots and their composites in biomedical theranostic fields such as medical imaging, gene therapy, chemotherapy, photothermal therapy, and photodynamic therapy are widely studied and reported. These researches are of great significance to the development of medical theranostic reagents based on carbon nanodots and their clinical advancement, and provide a novel drug system for the advancement of individualized, visualized, non-invasive, and minimally invasive diagnosis and treatment of major human diseases. This paper focuses on the design, construction and performance of carbon nanodots and their composites used in the field of theranostics. In addition, the research progress of the reported carbon nanodots based theranostic reagents in the biomedical field is discussed and summarized.
-
Key words:
- carbon nanodots /
- carbon nanodot complexes /
- theranostic agents /
- nanomedicine
-
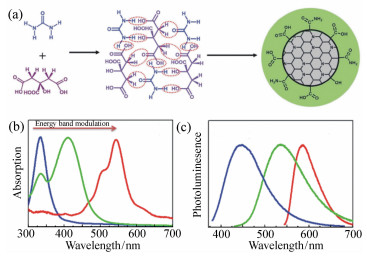
图 1 (a) 以柠檬酸和尿素为原料通过脱水和进一步碳化合成碳纳米点的示意图。通过调节碳基内核的共轭程度所获得的蓝光、绿光、红光发射的碳纳米点的吸收(b)和发射(c)光谱[20-21]
Figure 1. (a)A schematic illustration for the formation of carbon nanodots from citric acid and urea through dehydration and following carbonization. The absorption(b) and emission(c) spectra of blue, green and orange emissive carbon nanodots obtained by tuning the conjugation degree of carbon-based cores[20-21]
图 2 左:动态光散射测定的OCN新配置水分散液的数均粒径和滴涂在镍网上的无水态OCN的TEM图像。右:裸鼠前哨淋巴结的无损实时活体光声成像:激发光652 nm。右上:OCN刚注射后(2 min)采集获得的光声图像。右下:注射后210 min,图像对比度严重减弱[45]
Figure 2. Left: Number-averaged particle diameter from dynamic light scattering of as-synthesized OCN dispersed(0.2 M) in fresh water and anhydrous state TEM image drop-deposited on a nickel grid. Right: Non-invasive real-time in vivo PA imaging of SLN in a nude mouse: the laser was tuned to a wavelength of 650 nm. PA image acquired immediately (2 min) after the OCN injection(upper). The contrast is much weaker after 210 min post-injection(lower)[45]
图 3 在不同位置皮下注射GQDs后鼠明场(a)和红光(b)成像。激发波长为502~540 nm,荧光采集通道为695~775 nm。(c)经过各种处理后1天、9天、17天、25天鼠的照片。(PDT:GQDs+光照;C1:只注射GQDs;C2:只光照。)(d)经过各种处理后时间依赖肿瘤生长曲线(n=5),每组P<0.05[46](彩图见电子版)
Figure 3. (a)Bright-field image and (b)red-fluorescence image after subcutaneous injection of GQDs in different areas. The excitation wavelength was 502-540 nm, and the collected fluorescence channel was 695 775 nm. (c)Photographs of mice after various treatments on the 1st, 9th, 17th and 25th day(PDT:GQDs+light irradiation; C1:GQDs only; C2:light irradiation only.) (d)Time-dependent tumour growth curves(n=5) after different treatments. P < 0.05 for each group[46](color figures are available in electro-version)
图 4 (a) CNDs(左)和supra-CNDs(右)的溶液(上)和固体粉末(下)的光学照片;(b)CNDs和supra-CNDs的吸收光谱(紫外最大吸收处归一化);(c)不同浓度supra-CND水分散液在808 nm激光辐照(1 W/cm2)下与纯水对照的光热转换曲线[20]
Figure 4. (a)Optical images of CNDs(left) and supra-CNDs(right) in the solutions(upper) and the solid states(lower); (b)The absorption spectra (normalized at the absorption maxima in UV region) of CNDs and supra-CNDs; (c)Photothermal profile of supra-CND aqueous dispersions with different concentrations under 808 nm laser irradiation(1 W/cm2) in contrast to pure water[20]
图 6 (A) 细胞毒性和靶向性。(a)Gd@C-dots在不同PH值缓冲溶液中放置的光致发光强度变化。(b)Gd@C-dots随时间变化的Gd释放量。(c)MTT法测定U87MG细胞活性。(d)细胞靶向研究。(e)细胞颗粒T1-加权MR成像, 细胞与RGD-Gd@C-dots或Gd@C-dots共孵育。(B)(a)T1-加权断面MR成像,成像时间0, 10, 30, 45, 60 and 240 min。(b)T1-加权冠状MR成像。动物肿瘤注射RGD@Gd-dots后信号明显增强。(c)b中成像结果不同时间点信号变化。(d)肿瘤样品的免疫荧光组织学研究[52]
Figure 6. (A)Cytotoxicity and cell targeting. (a)Photoluminescence intensity change when Gd@C-dots were incubated in buffers of different pH values. (b)Gd release from Gd@C-dots over time. (c)Cell viability, evaluated by MTT assays with U87MG cells. (d)Cell targeting study. (e)T1-weighted MR images of cell pellets, where cells had been incubated with either RGD-Gd@C-dots or Gd@C-dots. (B) (a) T1-weighted transverse MR images. Images were acquired at 0, 10, 30, 45, 60 and 240 min. (b)T1-weighted coronal MR images. Significant signal enhancement was observed in tumors of animals injected with RGD@Gd-dots. (c)Relative signal change at different time points, based imaging results from b. (d)Immunofluorescence histology study with tumor samples[52]
图 9 实验设计示意图。(a)DOX上的胺基(-NH2)与CDs上的羧酸基团(-COOH)通过静电相互作用和氢键结合;(b)CD-DOX复合物传递至HepG2癌细胞和HL-7702正常细胞并伴随强绿光信号示踪。因为癌细胞内PH值低,CD-DOX复合物可将DOX释放至HepG2癌细胞,但不会释放到HL-7702正常细胞。基于GQD的FRET细胞核靶向传递系统实时监控药物释放过程的示意图[77]
Figure 9. Schematic illustration of the experimental design overview. (a)The amines(-NH2) on DOX bind with the carboxylic acid(-COOH) on CDs via electrostatic interactions or hydrogen bonding. (b)Delivery of CD DOX conjugates to HepG2 cancer cells and HL-7702 normal cells with strong green signal imaging tracking. The CD DOX conjugates are expected to release DOX in HepG2 cancer cells, but not HL-7702 normal liver cells, due to low pH in cancer cells.Schematic illustration of the GQD-based FRET system for nuclear-targeted delivery allowing for real-time monitoring the drug release process[77]
图 10 通过α-环糊精制备碳纳米点(CD)以及利用叶酸修饰碳纳米点负载酞菁锌(CD-PEG-FA/ZnPc)进行靶向光动力治疗的示意图[86]
Figure 10. Schematic illustration of the preparation of carbon nanodots(CD) from α-cyclodextrin and targeted photodynamic therapy with folic acid functionalized carbon nanodots loaded with zinc phthalocyanine(CD-PEG-FA/ZnPc)[86]
图 11 (a) 多功能FPC-NCs示意图。嵌入多孔碳壳的发光碳纳米点不仅能够作为共聚焦和双光子成像造影剂, 也能有效地将近红外光转换成热。同时, 大的中空腔和多孔碳壳可以通过FMC-NCs与DOX之间的超分子π堆积、氢键、和静电相互作用大量负载抗癌药(DOX)。因此,FPC-NCs可以将光热/化疗整合成一个纳米体系从而实现更高的疗效;(b)负载DOX的FPC-NCs化疗和近红外光照下光热/化学合并治疗示意图[89]
Figure 11. (a)Schematic illustration of multifunctional FPC-NCs. The fluorescent CDs embedded in the porous carbon shell can not only be used for confocal and two-photon imaging contrast, but also convert the NIR light to heat effectively. In addition, the large hollow cavity and porous carbon shell can provide a high loading capacity for anti-cancer drug(DOX) through the supramolecular π stacking, hydrogen bonding, and electrostatic interactions between DOX and FMC-NCs. Thus, the FPC-NCs can combine photothermal/chemotherapy into a single nano-object to provide high therapeutic efficacy; (b)Schematic illustration of the chemotherapy and combined chemo-photothermal treatment of the DOX-loaded FPC-NCs in the presence of NIR irradiation[89]
图 12 (a) 不同方法给药后C-dot-ZW800的尿富集。小鼠在异氟烷麻醉下,膀胱部位曝光,近红外成像图像在静脉(上)、肌肉(中)、皮下(下)注射前、后如图中标识的时间点获取。(b)(a)中ZW800荧光信号数值。(c)64Cu-C-dot经3种给药方式:左,静脉注射;中,肌肉注射;右,皮下注射后通过1h动态PET成像获得的代表性冠状位图像。(d)C-dot-ZW800经不同方式给药后的肿瘤富集情况[96]
Figure 12. (a)Urine accumulation of C-dot-ZW800 after different routes of injection. The mice were kept under isoflurane anesthesia, the bladder was exposed, and NIR images were acquired at the indicated time points before and after (top) iv injection, (middle) sc injection, and (bottom) im injection. (b)Quantification of the ZW800 fluorescence signal in (a). (c)Representative coronal images from 1 h dynamic PET imaging of 64Cu-C-dot after three routes of injection: left, iv injection; middle, sc injection; right, im injection. (d)Tumor uptake of C-dot-ZW800 after different routes of injection[96]
表 1 碳纳米点合成方法及特点总结
Table 1. Synthetic methods and properties of carbon nanodots
方法 原料 发射光谱 特点 自上而下 弧光放电、激光烧蚀、超声、电化学剥离、强酸氧化、水热、溶剂热等 炭黑、碳纤维、石墨、石墨烯、氧化石墨烯、碳纳米管等 蓝光-黄光,PLQYmax ~ 30% 石墨烯量子点,大部分需要表面钝化来提高荧光量子效率 自下而上 水热、溶剂热、微波热解、前聚体热解等 柠檬酸、尿素、葡萄糖、氨基酸、聚噻吩衍生物、苯胺衍生物等 紫外-可见-近红外光,PLQYmax ~ 90% 碳纳米点,尺寸、形貌易于调控,原料来源广,易掺杂 -
[1] LIM S Y, SHEN W, GAO Z. Carbon quantum dots and their applications[J]. Chemical Society Reviews, 2015, 44(1):362-381. doi: 10.1039/C4CS00269E [2] BAKER S N, BAKER G A. Luminescent carbon nanodots:emergent nanolights[J]. Angewandte Chemie International Edition, 2010, 49(38):6726-6744. doi: 10.1002/anie.200906623 [3] LI X, RUI M, SONG J, SHEN Z, et al.. Carbon and graphene quantum dots for optoelectronic and energy devices:a review[J]. Advanced Functional Materials, 2015, 25(31):4929-4947. doi: 10.1002/adfm.v25.31 [4] ZHAO A, CHEN Z, ZHAO C, et al.. Recent advances in bioapplications of C-dots[J]. Carbon, 2015, 85:309-327. doi: 10.1016/j.carbon.2014.12.045 [5] HOLA K, ZHANG Y, WANG Y, et al.. Carbon dots-emerging light emitters for bioimaging, cancer therapy and optoelectronics[J]. Nano Today, 2014, 9(5):590-603. doi: 10.1016/j.nantod.2014.09.004 [6] ZHENG X T, ANANTHANARAYANAN A, LUO K Q, et al.. Glowing graphene quantum dots and carbon dots:properties, syntheses, and biological applications[J]. Small, 2015, 11(14):1620-1636. doi: 10.1002/smll.v11.14 [7] LECROY G E, YANG S T, YANG F, et al.. Functionalized carbon nanoparticles:syntheses and applications in optical bioimaging and energy conversion[J]. Coordination Chemistry Reviews, 2016, 320-321:66-81. doi: 10.1016/j.ccr.2016.02.017 [8] DING C, ZHU A, TIAN Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging[J]. Accounts of Chemical Research, 2014, 47(1):20-30. doi: 10.1021/ar400023s [9] LUO P G, SAHU S, YANG S-T, et al.. Carbon "quantum" dots for optical bioimaging[J]. Journal of Materials Chemistry B, 2013, 1(16):2116-2127. doi: 10.1039/c3tb00018d [10] ZHANG J, YU S H. Carbon dots:large-scale synthesis, sensing and bioimaging[J]. Materials Today, 2016, 19(7):382-393. doi: 10.1016/j.mattod.2015.11.008 [11] FAN Z, LI S, YUAN F, et al.. Fluorescent graphene quantum dots for biosensing and bioimaging[J]. RSC Advances, 2015, 5(25):19773-19789. doi: 10.1039/C4RA17131D [12] MIAO P, HAN K, TANG Y, et al.. Recent advances in carbon nanodots:synthesis, properties and biomedical applications[J]. Nanoscale, 2015, 7(5):1586-1595. doi: 10.1039/C4NR05712K [13] WEGNER K D, HILDEBRANDT N. Quantum dots:bright and versatile in vitro and in vivo fluorescence imaging biosensors[J]. Chemical Society Reviews, 2015, 44(14):4792-4834. doi: 10.1039/C4CS00532E [14] LEMENAGER G, DE LUCA E, SUN Y P, et al.. Super-resolution fluorescence imaging of biocompatible carbon dots[J]. Nanoscale, 2014, 6(15):8617-8623. doi: 10.1039/C4NR01970A [15] GEORGAKILAS V, PERMAN J A., TUCEK J, et al.. Broad family of carbon nanoallotropes:classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures[J]. Chemical Reviews, 2015, 115(11):4744-4822. doi: 10.1021/cr500304f [16] XU X, RAY R, GU Y, et al.. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments[J]. Journal of the American Chemical Society, 2004, 126(40):12736-12737. doi: 10.1021/ja040082h [17] SUN Y P, ZHOU B, LIN Y, et al.. Quantum-sized carbon dots for bright and colorful photoluminescence[J]. Journal of the American Chemical Society, 2006, 128(24):7756-7757. doi: 10.1021/ja062677d [18] ZHU S, MENG Q, WANG L, et al.. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging[J]. Angewandte Chemie International Edition, 2013, 52(14):3953-3957. doi: 10.1002/anie.v52.14 [19] QU S, WANG X, LU Q, et al.. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots[J]. Angewandte Chemie International Edition, 2012, 51(49):12215-12218. doi: 10.1002/anie.v51.49 [20] LI D, HAN D, QU S N, et al.. Supra-(carbon nanodots) with a strong visible to near-infraredabsorption band and efficient photothermal conversion[J]. Light-Science & Applications, 2016, 5:e16120. https://www.researchgate.net/publication/304707406_Supra-carbon_nanodots_with_a_strong_visible_to_near-infrared_absorption_band_and_efficient_photothermal_conversion [21] QU S, ZHOU D, LI D, et al.. Toward efficient orange emissive carbon nanodots through conjugated sp(2)-domain controlling and surface charges engineering[J]. Advanced Materials, 2016, 28(18):3516-3521. doi: 10.1002/adma.201504891 [22] LU J, YEO P S E., GAN C K, et al.. Transforming C60 molecules into graphene quantum dots[J]. Nature Nanotechnology, 2011, 6(4):247-252. doi: 10.1038/nnano.2011.30 [23] YANG Y, WU D, HAN S, et al.. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft-hard template approach[J]. Chemical Communications, 2013, 49(43):4920-4922. doi: 10.1039/c3cc38815h [24] QU S, SHEN D, LIU X, et al.. Highly luminescent carbon-nanoparticle-based materials:factors influencing photoluminescence quantum Yield[J]. Particle & Particle Systems Characterization, 2014, 31(11):1175-1182. http://cn.bing.com/academic/profile?id=6718076f0004ed337be93ab035390b0b&encoded=0&v=paper_preview&mkt=zh-cn [25] DING H, YU S B, WEI J S, et al.. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism[J]. ACS Nano, 2016, 10(1):484-491. doi: 10.1021/acsnano.5b05406 [26] LECROY G E, SONKAR S K, YANG F, et al.. Toward structurally defined carbon dots as ultracompact fluorescent probes[J]. ACS Nano, 2014, 8(5):4522-4529. doi: 10.1021/nn406628s [27] ZHU L, CUI X, WU J, et al.. Fluorescence immunoassaybased on carbon dots as labels for the detection of hu -man immunoglobulin G[J]. Analytical Methods, 2014, 6(12):4430-4436. doi: 10.1039/C4AY00717D [28] ZHENG M, LIU S, LI J, et al.. Integrating oxaliplatin with highly luminescent carbon dots:an unprecedented theranostic agent for personalized medicine[J]. Advanced Materials, 2014, 26(21):3554-3560. doi: 10.1002/adma.v26.21 [29] LOU Q, QU S, JING P, et al.. Water-triggered luminescent "nano-bombs" based on supra-(carbon nanodots)[J]. Advanced Materials, 2015, 27(8):1389-1394. doi: 10.1002/adma.201403635 [30] 娄庆, 曲松楠.基于超级碳点的水致荧光"纳米炸弹"[J].中国光学, 2015, 8(1):91-98. http://www.chineseoptics.net.cn/CN/abstract/abstract9260.shtmlLOU Q, QU S N. Water triggered luminescent "nano-bombs" based on supra-carbon-nanodots[J]. Chinese Optics, 2015, 8(1):91-98.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9260.shtml [31] LIU W, LI C, REN Y, et al.. Carbon dots:surface engineering and applications[J]. Journal of Materials Chemistry B, 2016, 4(35):5772-5788. doi: 10.1039/C6TB00976J [32] SUDOLSKá M, DUBECKY M, SARKAR S, et al.. Nature of absorption bands in oxygen-functionalized graphitic carbon dots[J]. The Journal of Physical Chemistry C, 2015, 119(23):13369-13373. doi: 10.1021/acs.jpcc.5b04080 [33] WANG Y, KALYTCHUK S, ZHANG Y, et al.. Thickness-dependent full-color emission tunability in a flexible carbon dot ionogel[J]. The Journal Of Physical Chemistry Letters, 2014, 5(8):1412-1420. doi: 10.1021/jz5005335 [34] PAN L, SUN S, ZHANG A, et al.. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing[J]. Advanced Materials, 2015, 27(47):7782-7787. doi: 10.1002/adma.201503821 [35] ZHANG F, LIU F, WANG C, et al.. Effect of lateral size of graphene quantum dots on their properties and application[J]. ACS Applied Materials & Interfaces, 2016, 8(3):2104-2110. http://cn.bing.com/academic/profile?id=f9e20ca7361314cbe0b2e8bae0301ab8&encoded=0&v=paper_preview&mkt=zh-cn [36] VINCI J C, FERRER I M, SEEDHOUSE S J, et al. Hidden properties of carbon dots revealed after HPLC fractionation[J]. The Journal of Physical Chemistry Letters, 2013, 4(2):239-243. doi: 10.1021/jz301911y [37] PAN L, SUN S, ZHANG L, et al.. Near-infrared emissive carbon dots for two-photon fluorescence bioimaging[J]. Nanoscale, 2016, 8(39):17350-17356. doi: 10.1039/C6NR05878G [38] WANG X, CAO L, LU F, et al.. Photoinduced electron transfers with carbon dots[J]. Chemical Communications, 2009(25):3774-3776. doi: 10.1039/b906252a [39] JIANG K, ZHANG L, LU J, et al.. Triple-mode emission of carbon dots:applications for advanced anti-counterfeiting[J]. Angewandte Chemie International Edition, 2016, 55(25):7231-7235. doi: 10.1002/anie.201602445 [40] DENG Y, ZHAO D, CHEN X, et al.. Long lifetime pure organic phosphorescence based on water soluble carbon dots[J]. Chemical Communications, 2013, 49(51):5751-5753. doi: 10.1039/c3cc42600a [41] LI Q, ZHOU M, YANG Q, et al.. Efficient room-temperature phosphorescence from nitrogen-doped carbon dots in composite matrices[J]. Chemical Materials, 2016, 28(22):8221-8227. doi: 10.1021/acs.chemmater.6b03049 [42] CAO L, WANG X, MEZIANI M J, et al.. Carbon dots for multiphoton bioimaging[J]. Journal of the American Chemical Society, 2007, 129(37):11318-11319. doi: 10.1021/ja073527l [43] RUAN S, QIAN J, SHEN S, et al. A simple one-step method to prepare fluorescent carbon dots and their potential application in non-invasive glioma imaging[J]. Nanoscale, 2014, 6(17):10040-10047. doi: 10.1039/C4NR02657H [44] ZHENG M, RUAN S, LIU S, et al.. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells[J]. ACS Nano, 2015, 9(11):11455-11461. doi: 10.1021/acsnano.5b05575 [45] WU L, CAI X, NELSON K, et al.. A green synthesis of carbon nanoparticle from honey for real-time photoacoustic imaging[J]. Nano Research, 2013, 6(5):312-325. doi: 10.1007/s12274-013-0308-8 [46] GE J, LAN M, ZHOU B, et al.. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation[J]. Nature Communications, 2014, 5:4536. doi: 10.1038/ncomms5536 [47] GE J, JIA Q, LIU W, et al.. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice[J]. Advanced Materials, 2015, 27(28):4169-4177. doi: 10.1002/adma.v27.28 [48] GE J, JIA Q, LIU W, et al.. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo[J]. Advanced Healthcare Materials, 2016, 5(6):665-675. doi: 10.1002/adhm.201500720 [49] ZHENG M, LI Y, LIU S, et al.. One-pot to synthesize multifunctional carbon dots for near infrared fluorescence imaging and photothermal cancer therapy[J]. ACS Applied Materials & Interfaces, 2016, 8(36):23533-23541. http://cn.bing.com/academic/profile?id=3b5a295a63939846170a6164a756fdbf&encoded=0&v=paper_preview&mkt=zh-cn [50] SUN H, GAO N, DONG K, et al.. Graphene quantum dots-band-aids used for wound disinfection[J]. ACS Nano, 2014, 8(6):6202-6210. doi: 10.1021/nn501640q [51] 李欣远, 纪穆为, 王虹智, 等.近红外光热转换纳米晶研究进展[J].中国光学, 2017, 10(5):541-554. http://www.chineseoptics.net.cn/CN/abstract/abstract9545.shtmlLI X Y, JI M W, WANG H ZH, et al.. Research progress of near-infrared photothermal conversion nanocrystals[J]. Chinese Optics, 2017, 10(5):541-554.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9545.shtml [52] 苗少峰, 杨虹, 黄远辉, 等.光声成像研究进展[J].中国光学, 2015, 8(5):699-713. http://www.chineseoptics.net.cn/CN/abstract/abstract9338.shtmlMIAO SH F, YANG H, Huang Y H, et al.. Research progresses of photoacoustic imaging[J]. Chinese Optics, 2015, 8(5):699-713.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9338.shtml [53] 张砚, 汪源源, 李伟, 等.基于全变分法重建光声图像[J].光学精密工程, 2012, 20(1):204-212. http://www.opticsjournal.net/abstract.htm?id=OJ120214000041sYu2x5ZHANG Y, WANG Y Y, LI W, et al.. Reconstruction of photoacoustic image based on total variation[J]. Opt. Precision Eng., 2012, 20(1):204-212.(in Chinese) http://www.opticsjournal.net/abstract.htm?id=OJ120214000041sYu2x5 [54] SHI Y, PAN Y, ZHONG J, et al.. Facile synthesis of gadolinium(Ⅲ) chelates functionalized carbon quantum dots for fluorescence and magnetic resonance dual-modal bioimaging[J].Carbon, 2015, 93:742-750. doi: 10.1016/j.carbon.2015.05.100 [55] XU Y, JIA X H, YIN X B, et al.. Carbon quantum dot stabilized gadolinium nanoprobe prepared via a one-pot hydrothermal approach for magnetic resonance and fluorescence dual-modality bioimaging[J]. Analytical Chemistry, 2014, 86(24):12122-12129. doi: 10.1021/ac503002c [56] CHEN H, WANG G D, TANG W, et al.. Gd-encapsulated carbonaceous dots with efficient renal clearance for magnetic resonance imaging[J]. Advanced Materials, 014, 26(39):6761-6766. http://cn.bing.com/academic/profile?id=ac7d56e968bcb2d1fb0fc5d682aaeaaf&encoded=0&v=paper_preview&mkt=zh-cn [57] CHEN H, WANG G D, SUN X, et al.. Mesoporous silica as nanoreactors to prepare gd-encapsulated carbon dots of controllable sizes and magnetic properties[J]. Advanced Functional Materials, 2016, 26(22):3973-3982. doi: 10.1002/adfm.v26.22 [58] REN X, LIU L, LI Y, et al.. Facile preparation of gadolinium chelates functionalized carbon quantum dot based contrast agent for magnetic resonance/fluorescence multimodal imaging[J]. Journal of Materials Chemistry B, 2014, 2(34):5541-5549. doi: 10.1039/C4TB00709C [59] LIAO H, WANG Z, CHEN S, et al.. One-pot synthesis of gadolinium(Ⅲ) doped carbon dots for fluorescence/magnetic resonance bimodal imaging[J]. RSC Advances, 2015, 5(82):66575-66581. doi: 10.1039/C5RA09948J [60] SRIVASTAVA S, AWASTHI R, TRIPATHI D, et al.. Magnetic-nanoparticle-doped carbogenic nanocomposite:an effective magnetic resonance/fluorescence multimodal imaging probe[J]. Small, 2012, 8(7):1099-1109. doi: 10.1002/smll.201101863 [61] MARSHALL E. Gene therapy death prompts review of adenovirus vector[J]. Science, 1999, 286(5448):2244. doi: 10.1126/science.286.5448.2244 [62] SAKURAI H, KAWABATA K, SAKURAI F, et al.. Innate immune response induced by gene delivery vectors[J]. International Journal of Pharmaceutics, 2008, 354(1-2):9-15. doi: 10.1016/j.ijpharm.2007.06.012 [63] WANG L, WANG X, BHIRDE A, et al.. Carbon-dot-based two-photon visible nanocarriers for safe and highly efficient delivery of siRNA and DNA[J]. Advanced Healthcare Materials, 2014, 3(8):1203-1209. doi: 10.1002/adhm.v3.8 [64] LIU C, ZHANG P, ZHAI X, et al.. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence[J]. Biomaterials, 2012, 33(13):3604-3613. doi: 10.1016/j.biomaterials.2012.01.052 [65] KIM J, PARK J, KIM H, et al.. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-pDNA[J]. Biomaterials, 2013, 34(29):7168-7180. doi: 10.1016/j.biomaterials.2013.05.072 [66] HU L, SUN Y, LI S, et al.. Multifunctional carbon dots with high quantum yield for imaging and gene delivery[J]. Carbon, 2014, 67:508-513. doi: 10.1016/j.carbon.2013.10.023 [67] PIERRAT P, WANG R, KERESELIDZE D, et al. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers[J]. Biomaterials, 2015, 51:290-302. doi: 10.1016/j.biomaterials.2015.02.017 [68] KARTHIK S, SAHA B, GHOSH S K, et al.. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery[J]. Chemical Communications, 2013, 49(89):10471-10473. doi: 10.1039/c3cc46078a [69] WANG H, KE F, MARARENKO A, et al.. Responsive polymer-fluorescent carbon nanoparticle hybrid nanogels for optical temperature sensing, near-infrared light-responsive drug release, and tumor cell imaging[J]. Nanoscale, 2014, 6(13):7443-7452. doi: 10.1039/C4NR01030B [70] HE L, WANG T, AN J, et al.. Carbon nanodots@zeolitic imidazolate framework-8 nanoparticles for simultaneous pH-responsive drug delivery and fluorescence imaging[J]. Cryst. Eng. Comm., 2014, 16(16):3259-3263. doi: 10.1039/c3ce42506a [71] PANDEY S, MEWADA A, THAKUR M, et al.. Cysteamine hydrochloride protected carbon dots as a vehicle for the efficient release of the anti-schizophrenic drug haloperidol[J]. RSC Advances, 2013, 3(48):6290-26296. http://cn.bing.com/academic/profile?id=fca925f41926c87ac8c3e0248e976ce7&encoded=0&v=paper_preview&mkt=zh-cn [72] WANG Q, HUANG X, LONG Y, et al.. Hollow luminescent carbon dots for drug delivery[J]. Carbon, 2013, 59:192-199. doi: 10.1016/j.carbon.2013.03.009 [73] ZHOU L, LI Z, LIU Z, et al.. Luminescent carbon dot-gated nanovehicles for pH-triggered intracellular controlled release and imaging[J]. Langmuir, 2013, 29(21):6396-6403. doi: 10.1021/la400479n [74] MEWADA A, PANDEY S, THAKUR M, et al.. Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging[J]. Journal of Materials Chemistry B, 2014, 2(6):698-705. doi: 10.1039/C3TB21436B [75] WANG C, WU C, ZHOU X, et al.. Enhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dots[J]. Scientific Reports, 2013, 3:2852. doi: 10.1038/srep02852 [76] FAHMI M Z, CHEN J K, HUANG C C, et al.. Phenylboronic acid-modified magnetic nanoparticles as a platform for carbon dot conjugation and doxorubicin delivery[J]. Journal of Materials Chemistry B, 2015, 3(27):5532-5543. doi: 10.1039/C5TB00289C [77] ZENG Q. SHAO D, HE X, et al.. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo[J]. Journal of Materials Chemistry B, 2016, 4(30):5119-5126. doi: 10.1039/C6TB01259K [78] GONG X, ZHANG Q, GAO Y, et al.. Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging[J]. ACS Applied Materials & Interfaces, 2016, 8(18):11288-11297. http://cn.bing.com/academic/profile?id=a44fcbb97915c42f82e63e651c5b3a22&encoded=0&v=paper_preview&mkt=zh-cn [79] CHEN H, WANG Z, ZONG S, et al.. A graphene quantum dot-based FRET system for nuclear-targeted and real-time monitoring of drug delivery[J]. Nanoscale, 2015, 7(37):15477-15486. doi: 10.1039/C5NR03454J [80] XU X, ZHANG K, ZHAO L, et al.. Aspirin-based carbon dots, a good biocompatibility of material applied for bioimaging and anti-inflammation[J]. ACS Applied Materials & Interfaces, 2016, 8(48):32706-32716. http://cn.bing.com/academic/profile?id=98e6908cba7edb47edadb7f59b7605a8&encoded=0&v=paper_preview&mkt=zh-cn [81] DOLMANS D E J G J, FUKUMURA D, JAIN R K. Photodynamic therapy for cancer[J]. Nature Reviews Cancer, 2003, 3(5):380-387. doi: 10.1038/nrc1071 [82] HUANG P, LIN J, WANG X, et al.. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy[J]. Advanced Materials, 2012, 24(37):5104-5110. doi: 10.1002/adma.201200650 [83] BEACK S, KONG W H, JUNG H S, et al.. Photodynamic therapy of melanoma skin cancer using carbon dot-chlorin e6-hyaluronate conjugate[J]. Acta Biomaterialia, 2015, 26:295-305. doi: 10.1016/j.actbio.2015.08.027 [84] WANG J, ZHANG Z, ZHA S, et al.. Carbon nanodots featuring efficient FRET for two-photon photodynamic cancer therapy with a low fs laser power density[J]. Biomaterials, 2014, 35(34):9372-9381. doi: 10.1016/j.biomaterials.2014.07.063 [85] ZHOU L, ZHOU L, GE X, et al.. Multicolor imaging and the anticancer effect of a bifunctional silica nanosystem based on the complex of graphene quantum dots and hypocrellin A[J]. Chemical Commununications, 2015, 51(2):421-424. http://cn.bing.com/academic/profile?id=8201254b55e9f07c6dc5eb28a32805d8&encoded=0&v=paper_preview&mkt=zh-cn [86] CHOI Y, KIM S, CHOI M H, et al.. Highly biocompatible carbon nanodots for simultaneous bioimaging and targeted photodynamic therapy in vitro and in vivo[J]. Advanced Functional Materials, 2014, 24(37):5781-5789. doi: 10.1002/adfm.201400961 [87] VOGEL A, VENUGOPALAN V. Mechanisms of pulsed laser ablation of biological tissues[J]. Chemical Reviews, 2003, 103(2):577-644. doi: 10.1021/cr010379n [88] WANG H, SUN Y, YI J, et al.. Fluorescent porous carbon nanocapsules for two-photon imaging, NIR/pH dual-responsive drug carrier, and photothermal therapy[J]. Biomaterials, 2015, 53:117-126. doi: 10.1016/j.biomaterials.2015.02.087 [89] PANDEY S, THAKUR M, MEWADA A, et al.. Carbon dots functionalized gold nanorod mediated delivery of doxorubicin:tri-functional nano-worms for drug delivery, photothermal therapy and bioimaging[J]. Journal of Materials Chemistry B, 2013, 1(38):4972-4982. doi: 10.1039/c3tb20761g [90] YANG S T, WANG X, WANG H, et al.. Carbon dots as nontoxic and high-performance fluorescence imaging agents[J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2009, 113(42):18110-18114. doi: 10.1021/jp9085969 [91] TAO H, YANG K, MA Z, et al.. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite[J]. Small, 2012, 8(2):281-290. doi: 10.1002/smll.201101706 [92] WANG K, GAO Z, GAO G., et al.. Systematic safety evaluation on photoluminescent carbon dots[J]. Nanoscale Research Letters, 2013, 8(1):122. doi: 10.1186/1556-276X-8-122 [93] ZHENG X, SHAO D, LI J, et al.. Single and repeated dose toxicity of citric acid-based carbon dots and a derivative in mice[J]. RSC Advances, 2015, 5(111):91398-91406. doi: 10.1039/C5RA18391J [94] DAS B, DADHICH P, PAL P, et al.. Carbon nanodots from date molasses:new nanolights for the in vitro scavenging of reactive oxygen species[J]. Journal of Materials Chemistry B, 2014, 2(39):6839-6847. doi: 10.1039/C4TB01020E [95] LI S, GUO Z, ZHANG Y, et al.. Blood compatibility evaluations of fluorescent carbon dots[J]. ACS Applied Materials & Interfaces, 2015, 7(34):19153-19162. http://cn.bing.com/academic/profile?id=a5aeabc32185ce76b46c783b47d4821b&encoded=0&v=paper_preview&mkt=zh-cn [96] HUANG X, ZHANG F, ZHU L, et al.. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots[J]. ACS Nano, 2013, 7(7):5684-5693. doi: 10.1021/nn401911k -






 下载:
下载: