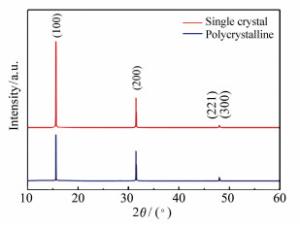
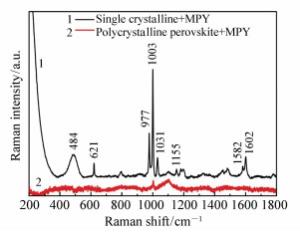
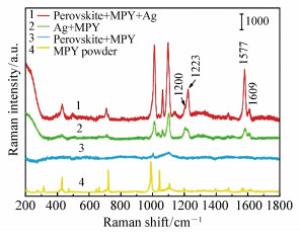
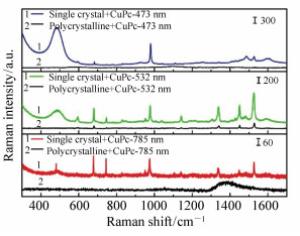
Charge transfer induced surface enhanced Raman scattering of single crystal and polycrystal perovskites
doi: 10.3788/CO.20191205.0952
-
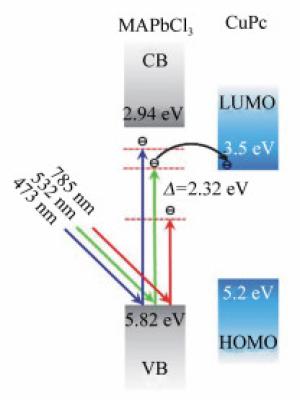
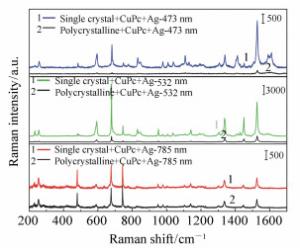
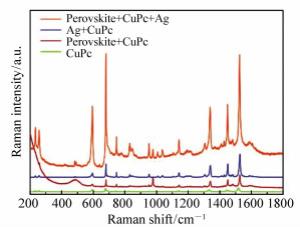
摘要: 近年来,钙钛矿作为一种新型的能源材料受到了众多学者的广泛关注。由于其具有较高的吸收系数、载流子迁移率以及扩散长度而被应用到光电器件中,例如:太阳能电池、光电探测器、场效晶体管以及发光二极管等。器件界面电荷转移过程则是影响钙钛矿材料性能的一个关键因素,在本工作中,利用表面增强拉曼光谱,研究了钙钛矿材料的电荷转移性质;制备了MAPbCl3钙钛矿单晶以及多晶薄膜,并在其表面沉积一层酞菁铜分子;随后,在酞菁铜表面再次沉积一层银膜。试图通过表面增强拉曼光谱(SERS)技术研究钙钛矿-钛菁铜界面的电荷转移过程以及表面银膜所产生的表面等离子体共振对于界面电荷转移及SERS性质的影响。研究结果表明,钙钛矿材料与钛菁铜分子能级匹配,且对于532 nm激发波长的激光具有良好的响应;532 nm激光能够诱导界面电荷转移过程的发生。同时,表面沉积的银膜可以进一步放大SERS信号。这主要是由于银膜的表面等离子体共振能够增强电荷分离,提高电荷转移效率,同时其表面产生的较强的电磁场,可以进一步增强钛菁铜分子的Raman信号强度。Abstract: The charge transfer(CT) process plays a key role in the operation of the optoelectronic device system so a better understanding of the interfacial CT property is greatly important. In this paper, Surface Enhanced Raman Scattering(SERS) was utilized to study the interfacial CT property between CuPc and perovskites(single crystal and polycrystalline). The Raman spectra of CuPc adsorbed on the perovskite surface was enhanced. The laser wavelength dependent SERS study indicates that this phenomenon is mainly arising from the CT from the VB band of perovskite to the LUMO band of the CuPc molecules. In comparison, the SERS signal of CuPc molecules adsorbed on a single crystal is much stronger than that on the polycrystalline perovskite. This result indicates that the defect status affects the enhancement ability of the materials. Further study shows that, after the decoration of a thin silver film, the SERS spectra of CuPc on both single crystal and polycrystalline perovskites are further enhanced. The extreme enhancement is not only due to the electromagnetic property of the silver film but also the fact that the SPR of the silver enhances the charge separation of the perovskite, which further promotes the CT process between the substrate and adsorbed molecules. The CT based SERS study shows great potential application value in the field of optoelectronic research.
-
Key words:
- SERS /
- perovskite /
- charge transfer /
- CuPc
-
Table 1. Band assignment of the CuPc molecules
CuPc Powder/cm-1 CuPc-Perovskite/cm-1 Bands Assignments 594 594 A1g 678 678 B1g, in plane full symmetric nonmetal bound N-M stretch and outer ring stretches 745 745 B2g, in plane ring symmetric N-M stretch 828 828 A1g, in plane full symmetric N-M stretch 951 951 1 036 1 037 B1g 1 140 1 140 A1g, in plane symmetric N-M-N bend 1 334 1 339 B1g, in plane full symmetric N-C stretch and ring C-C stretch 1 445 1 449 B2g, in plane ring symmetric outer ring C-C stretch 1 518 1 525 B2g, ring C-C stretch and in plane ring symmetric non metal bound N-C stretch -
[1] BURSCHKA J, PELLET N, MOON S J, et al.. Sequential deposition as a route to high-performance perovskite-sensitized solar cells[J]. Nature, 2013, 499(7458):316-319. doi: 10.1038/nature12340 [2] ZHAO Y X, ZHU K.Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications[J]. Chemical Society Reviews, 2016, 45(3):655-689. doi: 10.1039/C4CS00458B [3] TAN ZH K, MOGHADDAM R S, LAI M L, et al.. Bright light-emitting diodes based on organometal halide perovskite[J]. Nature Nanotechnology, 2014, 9(9):687-692. doi: 10.1038/nnano.2014.149 [4] YU W L, LI F, WANG H, et al.. Ultrathin Cu2O as an efficient inorganic hole transporting material for perovskite solar cells[J]. Nanoscale, 2016, 8(11):6173-6179. doi: 10.1039/C5NR07758C [5] SHI D, ADINOLFI V, COMIN R, et al.. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals[J]. Science, 2015, 347(6221):519-522. doi: 10.1126/science.aaa2725 [6] DONG Q F, FANG Y J, SHAO Y CH, et al.. Electron-hole diffusion lengths>175μm in solution-grown CH3NH3PbI3 single crystals[J]. Science, 2015, 347(6225):967-970. doi: 10.1126/science.aaa5760 [7] ZHOU H P, CHEN Q, LI G, et al.. Interface engineering of highly efficient perovskite solar cells[J]. Science, 2014, 345(6196):542-546. doi: 10.1126/science.1254050 [8] HAO F, STOUMPOS C C, CAO D H, et al.. Lead-free solid-state organic-inorganic halide perovskite solar cells[J]. Nature Photonics, 2014, 8(6):489-494. doi: 10.1038/nphoton.2014.82 [9] MA CH, SHI Y M, HU W J, et al.. Heterostructured WS2/CH3NH3PbI3photoconductors with suppressed dark current and enhanced photodetectivity[J]. Advanced Materials, 2016, 28(19):3683-3689. doi: 10.1002/adma.201600069 [10] LIU M ZH, JOHNSTON M B, SNAITH H J.Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467):395-398. doi: 10.1038/nature12509 [11] JEON N J, NOH J H, KIM Y C, et al.. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells[J]. Nature Materials, 2014, 13(9):897-903. doi: 10.1038/nmat4014 [12] XING G CH, MATHEWS N, SUN SH Y, et al.. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3[J]. Science, 2013, 42(6156):344-347. [13] STRANKS S D, EPERON G E, GRANCINI G, et al.. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber[J]. Science, 2013, 342(6156):341-344. doi: 10.1126/science.1243982 [14] MARCHIORO A, TEUSCHER J, FRIEDRICH D, et al.. Unravelling the mechanism of photoinduced charge transfer processes in lead iodide perovskite solar cells[J]. Nature Photonics, 2014, 8(3):250-255. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d63ca0b74c4db18927acc652d2f40017 [15] EDRI E, KIRMAYER S, MUKHOPADHYAY S, et al.. Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3-xClx perovskite solar cells[J]. Nature Communications, 2014, 5:3461. doi: 10.1038/ncomms4461 [16] BEGUM R, PARIDA M R, ABDELHADY A L, et al.. Engineering interfacial charge transfer in CsPbBr3 perovskite nanocrystals by heterovalent doping[J]. Journal of the American Chemical Society, 2017, 139(2):731-737. doi: 10.1021/jacs.6b09575 [17] LIU J X, LENG J, WU K F, et al.. Observation of internal photoinduced electron and hole separation in hybrid two-dimentional perovskite films[J]. Journal of the American Chemical Society, 2017, 139(4):1432-1435. doi: 10.1021/jacs.6b12581 [18] FLEISCHMANN M, HENDRA P J, MCQUILLAN A J. Ramanspectra of pyridine adsorbed at a silver electrode[J]. Chemical Physics Letters, 1974, 26(2):163-166. doi: 10.1016/0009-2614(74)85388-1 [19] LOMBARDI J R, BIRKE R L. A unified approach to surface-enhanced Raman spectroscopy[J]. The Journal of Physical Chemistry C, 2008, 112(14):5605-5617. doi: 10.1021/jp800167v [20] BELL S E J, SIRIMUTHU N M S. Quantitative surface-enhanced Raman spectroscopy[J]. Chemical Society Reviews, 2008, 37(5):1012-1024. doi: 10.1039/b705965p [21] PARK S, YANG P X, CORREDOR P, et al.. Transition metal-coated nanoparticle films:vibrational characterization with surface-enhanced Raman scattering[J]. Journal of the American Chemical Society, 2002, 124(11):2428-2429. doi: 10.1021/ja017406b [22] KNEIPP K, MOSKOVITS M, KNEIPP H. Surface-Enhanced Raman Scattering: Physics and Applications[M]. Berlin, Germany: Springer, 2006. [23] LOMBARDI J R, BIRKE R L. A unified view of surface-enhanced raman scattering[J]. Accounts of Chemical Research, 2009, 42(6):734-742. doi: 10.1021/ar800249y [24] YAMADA H, YAMAMOTO Y, TANI N. Surface-enhanced raman scattering(SERS) of adsorbed molecules on smooth surfaces of metals and a metal-oxide[J]. Chemical Physics Letters, 1982, 86(4):397-400. doi: 10.1016/0009-2614(82)83531-8 [25] YAMADA H, YAMAMOTO Y. Surface enhanced raman scattering(SERS) of chemisorbed species on various kinds of metals and semiconductors[J]. Surface Science, 1983, 134(1):71-90. doi: 10.1016/0039-6028(83)90312-6 [26] LING X, XIE L M, FANG Y, et al.. Can graphene be used as a substrate for raman enhancement?[J]. Nano Letters, 2010, 10(2):553-561. doi: 10.1021/nl903414x [27] LIVINGSTONE R, ZHOU X C, TAMARGO M C, et al.. Surface enhanced raman spectroscopy of pyridine on CdSe/ZnBeSe quantum dots grown by molecular beam epitaxy[J]. The Journal of Physical Chemistry C, 2010, 114(41):17460-17464. doi: 10.1021/jp105619m [28] JI W, KITAHAMA Y, XUE X X, et al.. Generation of pronounced resonance profile of charge-transfer contributions to surface-enhanced raman scattering[J]. The Journal of Physical Chemistry C, 2012, 116(3):2515-2520. doi: 10.1021/jp209947p [29] SUN ZH H, WANG CH X, YANG J X, et al.. Nanoparticle metal-semiconductor charge transfer in ZnO/PATP/Ag assemblies by surface-enhanced Raman spectroscopy[J]. The Journal of Physical Chemistry C, 2008, 112(15):6093-6098. doi: 10.1021/jp711240a [30] MAO ZH, SONG W, XUE X X, et al.. Multiphonon resonant raman scattering and photoinduced charge-transfer effects at ZnO-molecule interfaces[J]. The Journal of Physical Chemistry C, 2012, 116(51):26908-26918. doi: 10.1021/jp3092573 [31] WANG X L, WANG Y, SUI H M, et al.. Investigation of charge transfer in Ag/N719/TiO2 interface by surface-enhanced raman spectroscopy[J]. The Journal of Physical Chemistry C, 2016, 120(24):13078-13086. doi: 10.1021/acs.jpcc.6b03228 [32] TARAKESHWAR P, PALMA J L, FINKELSTEIN-SHAPIRO D, et al.. SERS as a probe of charge-transfer pathways in hybrid dye/molecule-metal oxide complexes[J]. The Journal of Physical Chemistry C, 2014, 118(7):3774-3782. doi: 10.1021/jp410725w [33] YU ZH, YU W L, XING J, et al.. Charge transfer effects on resonance-enhanced raman scattering for molecules adsorbed on single-crystalline perovskite[J]. ACS Photonics, 2018, 5(4):1619-1627. doi: 10.1021/acsphotonics.8b00152 [34] MACULAN G, SHEIKH A D, ABDELHADY A L, et al.. CH3NH3PbCl3 single crystals:inverse temperature crystallization and visible-blind UV-photodetector[J]. The Journal of Physical Chemistry Letters, 2015, 6(19):3781-3786. doi: 10.1021/acs.jpclett.5b01666 [35] BAIKIE T, BARROW N S, FANG Y A, et al.. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3(X=I, Br and Cl)[J].Journal of Materials Chemistry A, 2015, 3(17):9298-9307. doi: 10.1039/C5TA01125F [36] LING X, FANG W J, LEE Y H, et al.. Raman enhancement effect on two-dimensional layered materials:graphene, h-BN and MoS2[J]. Nano Letters, 2014, 14(6):3033-3040. doi: 10.1021/nl404610c [37] TAN Y, MA L N, GAO ZH B, et al.. Two-dimensional heterostructure as a platform for surface-enhanced raman scattering[J]. Nano Letters, 2017, 17(4):2621-2626. doi: 10.1021/acs.nanolett.7b00412 [38] BASOVA T V, KOLESOV B A. Raman spectra of copper phthalocyanin:experiment and calculation[J]. Journal of Structural Chemistry, 2000, 41(5):770-777. doi: 10.1023/A:1004802000669 [39] WANG M F, SPATARU T, LOMBARDI J R, et al.. Time resolved surface enhanced Raman scattering studies of 3-hydroxyflavone on a Ag electrode[J]. The Journal of Physical Chemistry C, 2007, 111(7):3044-3052. doi: 10.1021/jp0650937 [40] WANG M F, TESLOVA T, XU F, et al.. Raman and surface enhanced Raman scattering of 3-hydroxyflavone[J]. The Journal of Physical Chemistry C, 2007, 111(7):3038-3043. doi: 10.1021/jp062100i [41] KIM Y C, YANG T Y, JEON N J, et al.. Engineering interface structures between lead halide perovskite and copper phthalocyanine for efficient and stable perovskite solar cells[J]. Energy & Environmental Science, 2017, 10(10):2109-2116. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f2ae225853e3af2cc653c9b936edfb7a [42] NIU B J, WU L L, TANG W, et al.. Enhancement of near-band edge emission of Au/ZnO composite nanobelts by surface plasmon resonance[J]. CrystEngComm, 2011, 13(11):3678-3681. doi: 10.1039/c1ce05175j [43] SU Y H, TU S L, TSENG S W, et al.. Influence of surface plasmon resonance on the emission intermittency of photoluminescence from gold nano-sea-urchins[J]. Nanoscale, 2010, 2(12):2639-2646. doi: 10.1039/c0nr00330a [44] BABA A, AOKI N, SHINBO K, et al.. Grating-coupled surface plasmon enhanced short-circuit current in organic thin-film photovoltaic cells[J]. ACS Applied Materials & Interfaces, 2011, 3(6):2080-2084. doi: 10.1021/am200304x [45] SU Y H, KE Y F, CAI SH L, et al.. Surface plasmon resonance of layer-by-layer gold nanoparticles induced photoelectric current in environmentally-friendly plasmon-sensitized solar cell[J]. Light:Science & Applications, 2012, 1(6):e14. doi: 10.1038/lsa.2012.14 -





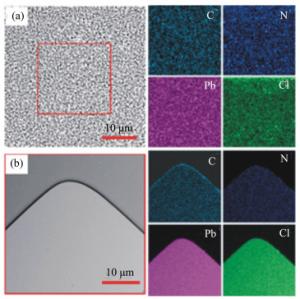
 下载:
下载: