Recent research progress on optimal design of halide perovskite photovoltaic materials
-
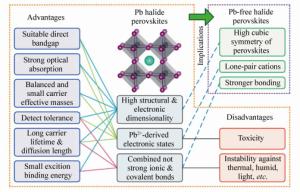
摘要: 以CH3NH3PbI3为代表的有机-无机杂化卤化物钙钛矿材料,具有独特优越的光电特性,例如与可见光谱基本匹配的禁带宽度、强的带边光吸收、平衡的双极性载流子输运、超长的载流子扩散距离以及合适的激子结合能等。从2009年到现在,基于钙钛矿材料构建的太阳能电池光电转化效率由最初的不足4%提升到了超过25%,结合低成本的溶液旋涂样品合成方法,使钙钛矿材料成为新型太阳能电池领域的研究热点。然而,高效率钙钛矿材料中铅元素引起的毒性,以及材料本身的不稳定性一直是阻碍太阳能电池及相关光电器件商业化的两大障碍,人们正在努力解决这些问题。在这篇综述中,详细总结了卤化物钙钛矿光伏材料的优化设计,包括结构式为AMX3的单钙钛矿,A2MM'X6的双钙钛矿,A2MX6的有序空位双钙钛矿,A'2An-1MnX3n+1的二维钙钛矿以及类钙钛矿材料(如A3M2X9)。通过材料优化设计,在一定程度上解决或改善了钙钛矿的材料稳定性和毒性问题,但光伏性能仍有待进一步优化提升。在此研究过程中,第一性原理高通量材料模拟在材料设计方面显示了预测能力,得到了与实验研究交互反馈、相互印证的结果。在综述研究进展的同时,进一步讨论了优化设计的新材料存在的问题,并展望了解决这些问题的潜在途径。Abstract: Organic-inorganic halide perovskite, as represented by CH3NH3PbI3, has been attracting increasing attention due to its advanced optoelectronic properties, such as suitable band gaps, high optical absorption, bipolar carrier conductivity, ultralong carrier diffusion length and appropriate exciton binding energy. At present, solar cells based on organic-inorganic halide perovskite have gained enormous significance and reached a power conversion efficiency exceeding 25%, which was less than 4% in 2009. In addition, combined with the low-cost solution spin-coating method for sample preparation, perovskite materials have become the focus of research in the field of novel solar cells. Researchers have been addressing the key challenges facing lead halide perovskites, including their stability and toxicity issues. In this paper, research progress on the optimal designs of halide perovskite photovoltaic materials is reviewed in detail, including single(AMX3), double(A2MM'X6), ordered-vacancy double(A2MX6), 2D(A'2An-1MnX3n+1) perovskites and perovskite-like(A3M2X9) materials. Through rational design, the material stability and toxicity of perovskites have been solved or improved to some extent but the photovoltaic performance has yet to be further optimized. In this research, the first-principles of high-throughput material simulation showed predictive ability in material design. The results of interactive feedback and mutual verification with experimental research were obtained. In addition, the problems introduced by new materials in rational designs are discussed and a powerful method for addressing these problems is proposed.
-
Key words:
- solar cell /
- halide perovskites /
- optoelectronic conversion /
- materials by design
-
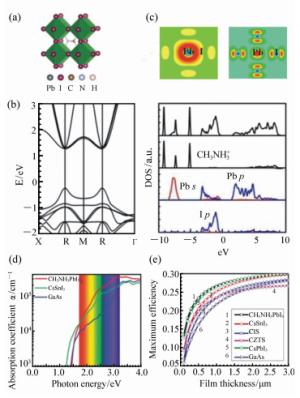
图 2 (a) MAPbI3立方相的晶体结构;(b)MAPbI3的能带结构(纵坐标每小格为0.2 eV)和态密度,从上到下分别为总的态密度,CH3NH3+,Pb,I的分波态密度[50];(c)MAPbI3的价带和导带的部分电荷密度[50];(d)理论计算的MAPbI3,CsSnI3,CsPbI3的光吸收系数[24];(e)MAPbI3与几种常见光伏材料的光电转换效率随层厚度的关系的比较[24]
Figure 2. (a)Crystal structure of MAPbX3 perovskites(MA=methylammonium; X=I, Br, or Cl). (b)The band structure (0.2 eV per division of CH3NH3PbI3 and total DOS and CH3NH3+, Pb, I partial DOS, respectively[50]. (c)The partial charge densities at conduction band minimum(CBM) and valence band maximum(VBM) [50]. (d)The optical absorptions of MAPbI3, CsSnI3 and GaAs[24]. (e)Calculated maximum efficiencies of halide perovskites, CIS, CZTS, and GaAs as a function of film thickness[24]
图 3 (a) MAPbI3的缺陷形成能示意图[50]。(b)中性MAPbI3中点缺陷的跃迁能级,红色和蓝色分别代表受主和施主,括号里表示的是从小到大排序的缺陷形成能。MA+表示CH3NH3+[50]
Figure 3. (a)The formation energies of intrinsic point defects in CH3NH3PbI3[50]. (b)Calculated transition energy levels of point defects in CH3NH3PbI3[50].The formation energies of neutral defects are shown in parenthesis. The red and blue lines represent the acceptors and donors, respectively[50]
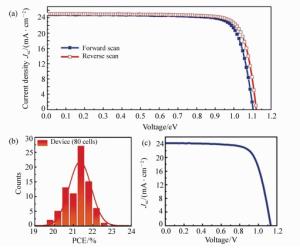
图 4 (a) 面积仅为0.095cm2太阳能电池材料的正向和反向扫描的电流-电压(J-V)曲线[62]。(b)80个太阳能电池器件功率转化效率的直方图[62]。(c)面积为1.0 cm2的电流-电压曲线(J-V)[62]
Figure 4. (a)J-V curves of a small PSC(0.095 cm2) in forward- and reverse-scan modes and the corresponding photovoltaic parameters[62]. (b)Histogram of the average power conversion efficiency determined for 80 PSC devices[62]. (c)J-V curve for a large PSC(1.0 cm2) plotted as the average of the reverse- and forward-scan modes and the corresponding photovoltaic parameters[62]
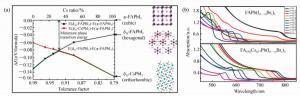
图 6 (a) 计算所得到的FA1-xCsxPbI3合金不同Cs比率α相和δ相的能量差随容差因子t的变化[25]。(b)FAPb(I1-xBrx)3和FA0.83Cs0.17(I1-xBrx)3的不同组分的光吸收系数[77]
Figure 6. (a)Calculated energy difference between α-phase and different δ-phases for FA1-xCsxPbI3alloys with different Cs ratios[25]. (b)UV-vis absorption spectra of FAPb(I1-xBrx)3 and FA0.83Cs0.17Pb(I1-xBrx)3 thin films(FA=formamidinium)[77]
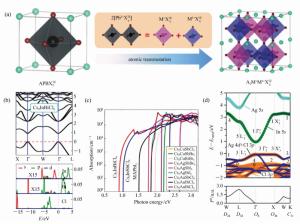
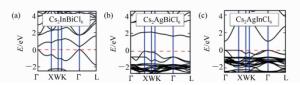
图 7 (a) 由单钙钛矿演化成双钙钛矿的过程[113]。(b)Cs2InBiCl6能带结构和轨道投影态密度[113]。(c)理论计算的A2M+M3+X6Ⅶ钙钛矿最佳光谱[113]。(d)Cs2[AgIn]Cl6的能带投影和跃迁矩阵元[126]
Figure 7. (a)Space of candidate A2M+M3+X6Ⅶ perovskites for materials screening: left panel shows adopted double-perovskite structure, and right panel shows schematic idea of atomic transmutation[113]. (b)Electronic band structures and orbital-projected density of states for Cs2InBiCl6[113]. (c)Calculated absorption spectra of selected optimal A2M+M3+X6Ⅶ perovskites[113]. (d)Band structure and transition matrix elements for Cs2AgInCl6[126]
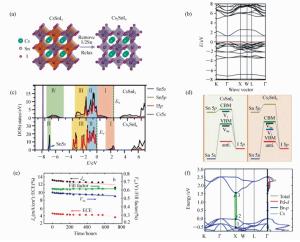
图 9 (a) Cs2SnI6的晶体结构[136];(b)Heyd-Scuseria-Ernzerhof(HSE)泛函计算的Cs2SnI6的能带结构[137];(c)理论计算的CsSnI3(上)和Cs2SnI6(下)的投影态密度[136];(d)CsSnI3(左)和Cs2SnI6(右)的简易能级示意图[136];(e)Cs2SnI6的固态染料敏化太阳能电池的以一定间隔时间测量的长期的光伏参数[138];(f)Cs2PdBr6的电子能带结构和投影态密度。黑点(1)和(2)表示X点的最高和第二高占据态;(3)表示X点最低未占据态[140]
Figure 9. (a)Crystal structure of Cs2SnI6, which is obtained by removing a half of the Sn atoms at intervals[136]. (b)Band structure of Cs2SnI6 calculated with HSE06[137]. (c)Total and projected densities of states(DOSs/PDOSs) of CsSnI3(top panel), and Cs2SnI6(bottom panel)[136]. (d)Simplified energy diagrams depicting the formation of VBM, CBM, and donor-/acceptor-like defects in (left)CsSnI3 and (right)Cs2SnI6[136]. (e) Long-term measurement of the parameters taken at regular intervals as a function of time[138]. (f)Electronic band structure of Cs2PdBr6[140]
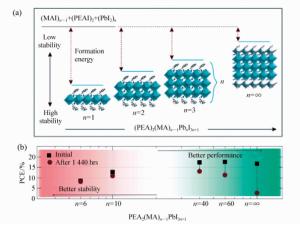
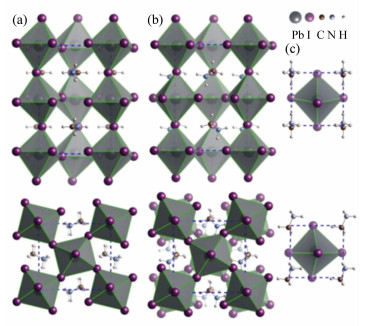
图 10 (a)(PEA)2(MA)n-1PbnI3n+1从二维(n=1)到三维(n=∞)的晶体结构变化[115]。(b)(PEA)2(MA)n-1PbnI3n+1二维钙钛矿和三维钙钛矿的稳定性和效率的比较[115]
Figure 10. (a)Unit cell structure of (C8H9NH3)2(CH3NH3)n-1PbnI3n+1 perovskites with different n values, showing the evolution of dimensionality from 2D(n=1) to 3D(n=∞)[115]. (b)Device performance as a function of n value, which shows that increased performance was achieved with increased n value; however, in the meantime, stability was decreased[115]
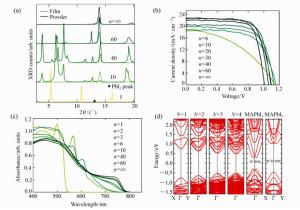
图 11 (a) 不同层(PEA)2(MA)Pbn-1I3n+1薄膜结构低角度衍射的XRD图[115]。(b)不同层(PEA)2(MA)n-1PbI3n+1薄膜的光吸收谱[115]。(c)不同层(PEA)2(MA)n-1PbnI3n+1薄膜的电流-电压曲线(J-V)[115]。(d)通过DFT+SOC方法计算的(PEA)2(MA)n-1I3n+1(n=1, 2, 3, 4)的能带结构和MAPbI3是否考虑SOC计算的能带结构的比较[150]
Figure 11. (a)Low-diffraction-angle region of XRD spectra[115].(b)Absorption spectra of the perovskite films with different n values[115]. (c)J-V curve for champion perovskite device with different n values[115]. (d)Calculated band structures of (PEA)2PbI4(N=1-4) by DFT-PBE+SOC, and those of MAPbI3 by DFT with and without SOC[150]
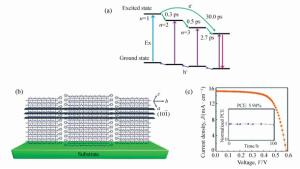
图 12 (a) n=3准二维钙钛矿薄膜中载流子转移的示意图[151]。(b)高度定向二维Sn基钙钛矿薄膜示意图[153]。(c)高度定向(PEA)2(FA)8Sn9I28的最高性能器件的电流密度-电压(J-V)特性。插图显示设备在手套箱中存储超过100小时的标准化的太阳能转换效率[153]
Figure 12. (a)Schematic of carrier transfer in the n=3 perovskite film. The electron transfers from small-n to large-n perovskite phases, and the hole transfers from large-n to small-n perovskite phases[151]. (b)Schematic illustration of the (101) plane of a (PEA)2(FA)8Sn9I28(n=9) 2D perovskite crystal[153]. (c)Current-density voltage(J-V) characteristics of the highest-performance device based on highly oriented (PEA)2(FA)8Sn9I28. The inset indicates the normalized PCE of the device stored in a glove box for over 100 h[153]
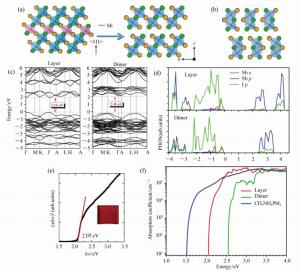
图 13 (a) Cs3Sb3I9去除Sb后得到Cs3Sb2I9为代表的层状相的结构[155]。(b)Cs3Sb2I9为代表的二聚体相的结构[155]。(c)HSE计算的层状相和二聚体相的能带结构[155]。(d)层状相和二聚体相的态密度[155]。(e)用于确定层状相Cs3Sb2I9的带隙的光学吸收(插图表示薄膜样品)[155]。(f)理论计算的Cs3Sb2I9层状相和二聚体相的吸收系数和MAPbI3的比较[155]
Figure 13. (a)Removal of every third Sb layer along the <111> direction of the perovskite structure results in the 2D layered modification of Cs3Sb2I9[155]. (b)2D layered modification of Cs3Sb2I9[155]. (c)HSE calculated band structures of Cs3Sb2I9 in layered and dimer modifications[155]. (d)Partial density of states(PDOS) plots of the layered and dimer modifications of Cs3Sb2I9[155]. (e)Band gap of the layered modification of Cs3Sb2I9(inset shows a thin film) was calculated to be 2.05 eV from absorbance data using the Tauc relation[155]. (f)Calculated absorption coefficients for Cs3Sb2I9 with the layered and dimer structures as compared to that of CH3NH3PbI3[155]
表 1 不同类型卤化物双钙钛矿的总结[39]
Table 1. Summary of different categories of halide double perovskites
A2B(Ⅰ)B(Ⅲ)X6 Optoelectronic properties Synthesized compounds (Potential)Applications Type Ⅰ: direct bandgap (MA)2TlBiBr6 (solar cell) s2+s2 suitable bandgap values (light-emission device) strong light absorption high electronic dimensionality expected defect tolerance Type Ⅱ: indirect bandgap Cs2AgBiCl6 solar cell s0+s2 large bandgap values Cs2AgBiBr6 X-ray detector reduced electronic (MA)2AgBiBr6 photocatalysis dimensionality (MA)2AgBiI6 (X-ray imaging) long carrier lifetime Cs2AgSbCl6 not good carrier transport (MA)2AgSbI6 (MA)2KBiCl6 Cs2NaBiCl6 Type Ⅲ: direct bandgap Cs2AgInCl6 photodetector s0+s0 dipole-forbidden transition (MA)2KGdCl6 laser large bandgap values (MA)2KYCl6 light-emission reduced electronic Cs2NaGaF6 device dimensionality Vacancy-ordered direct bandgap Cs2SnI6 solar cell strong light absorption Cs2PdBr6 light-emission existence of deep mid-gap Cs2Ti[Br/I]6 device detects Cs2TeI6 (X-ray imaging) not good carrier transport -
[1] CHAPIN D M, FULLER C S, PEARSON G L. A new silicon p-n junction photocell for converting solar radiation into electrical power[J]. Journal of Applied Physics, 1954, 25(5):676-677. doi: 10.1063/1.1721711 [2] WANG Y, JIN Y H, DUAN Y H, et al.. Fe3O4 quantum dots on 3D-framed graphene aerogel as an advanced anode material in lithium-ion batteries[J]. Ionics, 2017, 23(8):2005-2011. doi: 10.1007/s11581-017-2044-7 [3] O'REGAN B, GRÄTZEL M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films[J]. Nature, 1991, 353(6346):737-740. doi: 10.1038/353737a0 [4] MATHEW S, YELLA A, GAO P, et al.. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers[J]. Nature Chemistry, 2014, 6(3):242-247. doi: 10.1038/nchem.1861 [5] HODES G. Perovskite-based solar cells[J]. Science, 2013, 342(6156):317-318. doi: 10.1126/science.1245473 [6] KOJIMA A, TESHIMA K, SHIRAI Y, et al.. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells[J]. Journal of the American Chemical Society, 2009, 131(17):6050-6051. doi: 10.1021/ja809598r [7] TAN F R, TAN H R, SAIDAMINOV M I, et al.. In situ back-contact passivation improves photovoltage and fill factor in perovskite solar cells[J]. Advanced Materials, 2019, 31(14):1807435. doi: 10.1002/adma.201807435 [8] BAUHUIS G J, MULDER P, HAVERKAMP E J, et al.. 26.1% thin-film GaAs solar cell using epitaxial lift-off[J]. Solar Energy Materials and Solar Cells, 2009, 93(9):1488-1491. doi: 10.1016/j.solmat.2009.03.027 [9] SONG B G, AHN H Y, PARK B I, et al.. Effects of compression and controlled selenization on powder-fabricated Cu(In, Ga)Se2 thin films[J]. Applied Surface Science, 2019, 475:158-161. doi: 10.1016/j.apsusc.2018.12.196 [10] BRITT J, FEREKIDES C. Thin-film CdS/CdTe solar cell with 15.8% efficiency[J]. Applied Physics Letters, 1993, 62(22):2851-2852. doi: 10.1063/1.109629 [11] TODOROV T K, TANG J, BAG S, et al.. Beyond 11% efficiency:characteristics of state-of-the-art Cu2ZnSn(S, Se)4 solar cells[J]. Advanced Energy Materials, 2013, 3(1):34-38. doi: 10.1002/aenm.201200348 [12] XING G CH, MATHEWS N, SUN SH Y, et al.. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3[J]. Science, 2013, 342(6156):344-347. doi: 10.1126/science.1243167 [13] GIORGI G, FUJISAWA J I, SEGAWA H, et al.. Small photocarrier effective masses featuring ambipolar transport in methylammonium lead iodide perovskite:a density functional analysis[J]. The Journal of Physical Chemistry Letters, 2013, 4(24):4213-4216. doi: 10.1021/jz4023865 [14] FROHNA K, DESHPANDE T, HARTER J, et al.. Inversion symmetry and bulk Rashba effect in methylammonium lead iodide perovskite single crystals[J]. Nature Communications, 2018, 9:1829. doi: 10.1038/s41467-018-04212-w [15] STRANKS S D, EPERON G E, GRANCINI G, et al.. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber[J]. Science, 2013, 342(6156):341-344. doi: 10.1126/science.1243982 [16] WEHRENFENNIG C, EPERON G E, JOHNSTON M B, et al.. High charge carrier mobilities and lifetimes in organolead trihalide perovskites[J]. Advanced Materials, 2014, 26(10):1584-1589. doi: 10.1002/adma.201305172 [17] EDRI E, KIRMAYER S, MUKHOPADHYAY S, et al.. Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3-xClx perovskite solar cells[J]. Nature Communications, 2014, 5:3461. doi: 10.1038/ncomms4461 [18] MIYATA A, MITIOGLU A, PLOCHOCKA P, et al.. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic-inorganic tri-halide perovskites[J]. Nature Physics, 2015, 11(7):582-587. doi: 10.1038/nphys3357 [19] BAUMANN A, VÄTH S, RIEDER P, et al.. Identification of trap states in perovskite solar cells[J]. The Journal of Physical Chemistry Letters, 2015, 6(12) 2350-2354. doi: 10.1021/acs.jpclett.5b00953 [20] DUAN H S, ZHOU H P, CHEN Q, et al.. The identification and characterization of defect states in hybrid organic-inorganic perovskite photovoltaics[J]. Physical Chemistry Chemical Physics, 2015, 17(1):112-116. doi: 10.1039/C4CP04479G [21] WANG J T W, WANG ZH P, PATHAK S, et al.. Efficient perovskite solar cells by metal ion doping[J]. Energy & Environmental Science, 2016, 9(9):2892-2901. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0f10449972987006d2c6d75da5894f1d [22] ZHAO D W, YU Y, WANG CH L, et al.. Low-bandgap mixed tin-lead iodide perovskite absorbers with long carrier lifetimes for all-perovskite tandem solar cells[J]. Nature Energy, 2017, 2(4):17018. doi: 10.1038/nenergy.2017.18 [23] XIAO Z W, YAN Y F. Progress in theoretical study of metal halide perovskite solar cell materials[J]. Advanced Energy Materials, 2017, 7(22):1701136. doi: 10.1002/aenm.201701136 [24] YIN W J, SHI T T, YAN Y F. Unique properties of halide perovskites as possible origins of the superior solar cell performance[J]. Advanced Materials, 2014, 26(27):4653-4658. doi: 10.1002/adma.201306281 [25] LI Z, YANG M J, PARK J S, et al.. Stabilizing perovskite structures by tuning tolerance factor:formation of formamidinium and cesium lead iodide solid-state alloys[J]. Chemistry of Materials, 2016, 28(1):284-292. doi: 10.1021/acs.chemmater.5b04107 [26] ZHANG H, WANG H, WILLIAMS S T, et al.. SrCl2 derived perovskite facilitating a high efficiency of 16% in hole-conductor-free fully printable mesoscopic perovskite solar cells[J]. Advanced Materials, 2017, 29(15):1606608. doi: 10.1002/adma.201606608 [27] LI L, LIU N, XU Z Q, et al.. Precise composition tailoring of mixed-cation hybrid perovskites for efficient solar cells by mixture design methods[J]. ACS Nano, 2017, 11(9):8804-8813. doi: 10.1021/acsnano.7b02867 [28] PELLET N, GAO P, GREGORI G, et al.. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting[J]. Angewandte Chemie International Edition, 2014, 53(12):3151-3157. doi: 10.1002/anie.201309361 [29] LEE M M, TEUSCHER J, MIYASAKA T, et al.. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites[J]. Science, 2012, 338(6107):643-647. doi: 10.1126/science.1228604 [30] NOH J H, IM S H, HEO J H, et al.. Chemical management for colorful, efficient, and stable inorganic organic hybrid nanostructured solar cells[J]. Nano Letters, 2013, 13(4):1764-1769. doi: 10.1021/nl400349b [31] OGOMI Y, MORITA A, TSUKAMOTO S, et al.. CH3NH3SnxPb(1-x)I3 perovskite solar cells covering up to 1060 nm[J]. The Journal of Physical Chemistry Letters, 2014, 5(6):1004-1011. doi: 10.1021/jz5002117 [32] SALIBA M, MATSUI T, SEO J Y, et al.. Cesium-containing triple cation perovskite solar cells:improved stability, reproducibility and high efficiency[J]. Energy & Environmental Science, 2016, 9(6):1989-1997. https://www.ncbi.nlm.nih.gov/pubmed/27478500 [33] GRÄTZEL M. The light and shade of perovskite solar cells[J]. Nature Materials, 2014, 13(9):838-842. doi: 10.1038/nmat4065 [34] SAPAROV B, MITZI D B. Organic-inorganic perovskites:Structural versatility for functional materials design[J]. Chemical Reviews, 2016, 116(7) 4558-4596. doi: 10.1021/acs.chemrev.5b00715 [35] HU H, DONG B H, ZHANG W. Low-toxic metal halide perovskites:opportunities and future challenges[J]. Journal of Materials Chemistry A, 2017, 5(23):11436-11449. doi: 10.1039/C7TA00269F [36] MANSER J S, CHRISTIANS J A, KAMAT P V. Intriguing optoelectronic properties of metal halide perovskites[J]. Chemical Reviews, 2016, 116(21):12956-13008. doi: 10.1021/acs.chemrev.6b00136 [37] YIN W J, YANG J H, KANG J, et al.. Halide perovskite materials for solar cells:a theoretical review[J]. Journal of Materials Chemistry A, 2015, 3(17):8926-8942. doi: 10.1039/C4TA05033A [38] XU Q L, YANG D W, LV J, et al.. Perovskite solar absorbers:materials by design[J]. Small Methods, 2018, 2(5):1700316. doi: 10.1002/smtd.201700316 [39] ZHAO X G, YANG D W, REN J CH, et al.. Rational design of halide double perovskites for optoelectronic applications[J]. Joule, 2018, 2(9):1662-1673. doi: 10.1016/j.joule.2018.06.017 [40] LIANG L SH, GAO P. Lead-free hybrid perovskite absorbers for viable application:can we eat the cake and have it too?[J]. Advanced Science, 2018, 5(2):1700331. doi: 10.1002/advs.201700331 [41] XIAO Z W, MENG W W, WANG J B, et al.. Searching for promising new perovskite-based photovoltaic absorbers:the importance of electronic dimensionality[J]. Materials Horizons, 2017, 4(2):206-216. https://www.onacademic.com/detail/journal_1000042618044499_f68a.html [42] XIAO Z W, SONG ZH N, YAN Y F. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives[J]. Advanced Materials, 2019:1803792, doi: 10.1002/adma.201803792. [43] SALIBA M, MATSUI T, DOMANSKI K, et al.. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance[J]. Science, 2016, 354(6309):206-209. doi: 10.1126/science.aah5557 [44] LI C, LU X G, DING W ZH, et al.. Formability of ABX3(X=F, Cl, Br, I) halide perovskites[J]. Acta Crystallographica Section B, 2008, 64(6):702-707. doi: 10.1107/S0108768108032734 [45] EPERON G E, PATERN G M, SUTTON R J, et al.. Inorganic caesium lead iodide perovskite solar cells[J]. Journal of Materials Chemistry A, 2015, 3(39):19688-19695. doi: 10.1039/C5TA06398A [46] STOUMPOS C C, MALLIAKAS C D, KANATZIDIS M G. Semiconducting tin and lead iodide perovskites with organic cations:phase transitions, high mobilities, and near-infrared photoluminescent properties[J]. Inorganic Chemistry, 2013, 52(15):9019-9038. doi: 10.1021/ic401215x [47] BAIKIE T, FANG Y N, KADRO J M, et al.. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications[J]. Journal of Materials Chemistry A, 2013, 1(18):5628-5641. doi: 10.1039/c3ta10518k [48] BRIVIO F, FROST J M, SKELTON J M, et al.. Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide[J]. Physical Review B, 2015, 92(14):144308. doi: 10.1103/PhysRevB.92.144308 [49] MOTTA C, EL-MELLOUHI F, KAIS S, et al.. Revealing the role of organic cations in hybrid halide perovskite CH3NH3PbI3[J]. Nature Communications, 2015, 6:7026. doi: 10.1038/ncomms8026 [50] YIN W J, SHI T T, YAN Y F. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber[J]. Applied Physics Letters, 2014, 104(6):063903. doi: 10.1063/1.4864778 [51] ZHENG F, TAN L Z, LIU SH, et al.. Rashba spin orbit coupling enhanced carrier lifetime in CH3NH3PbI3[J]. Nano Letters, 2015, 15(12):7794-7800. doi: 10.1021/acs.nanolett.5b01854 [52] GAO W W, GAO X, ABTEW T A, et al.. Quasiparticle band gap of organic-inorganic hybrid perovskites:crystal structure, spin-orbit coupling, and self-energy effects[J]. Physical Review B, 2016, 93(8):085202. doi: 10.1103/PhysRevB.93.085202 [53] EL JANI B, GIBART P, PORTAL J C, et al.. Effective masses in Sn-doped Ga1-xAlxAs(x < 0.3) determined by the Shubnikov-de Haas effect[J]. Journal of Applied Physics, 1985, 58(9):3481-3484. doi: 10.1063/1.335771 [54] FAN ZH, SUN K, WANG J. Perovskites for photovoltaics:a combined review of organic-inorganic halide perovskites and ferroelectric oxide perovskites[J]. Journal of Materials Chemistry A, 2015, 3(37):18809-18828. doi: 10.1039/C5TA04235F [55] KIM H S, LEE C R, IM J H, et al.. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%[J]. Scientific Reports, 2012, 2:591. doi: 10.1038/srep00591 [56] LIU M ZH, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467):395-398. doi: 10.1038/nature12509 [57] GREEN M A, MERY K, ISHIKAWA Y, et al.. Solar cell efficiency tables(Version 45)[J]. Progress in Photovoltaics:Research and Applications, 2015, 23(1):1-9. doi: 10.1002/pip.2573 [58] YANG ZH, SURRENTE A, GALKOWSKI K, et al.. Unraveling the exciton binding energy and the dielectric constant in single-crystal methylammonium lead triiodide perovskite[J]. The Journal of Physical Chemistry Letters, 2017, 8(8):1851-1855. doi: 10.1021/acs.jpclett.7b00524 [59] DU M H. Efficient carrier transport in halide perovskites:theoretical perspectives[J]. Journal of Materials Chemistry A, 2014, 2(24):9091-9098. doi: 10.1039/C4TA01198H [60] TANAKA K, AKAHASHI T, BAN T, et al.. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH3NH3PbBr3 CH3NH3PbI3[J]. Solid State Communications, 2003, 127(9-10):619-623. doi: 10.1016/S0038-1098(03)00566-0 [61] GALKOWSKI K, MITIOGLU A, MIYATA A, et al.. Determination of the exciton binding energy and effective masses for methylammonium and formamidinium lead tri-halide perovskite semiconductors[J]. Energy & Environmental Science, 2016, 9(3):962-970. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=85de4f0693dbb1e7d581b8c09cfd05d9 [62] YANG W S, PARK B W, JUNG E H, et al.. Iodide management in formamidinium-lead-halide based perovskite layers for efficient solar cells[J]. Science, 2017, 356(6345):1376-1379. doi: 10.1126/science.aan2301 [63] ABATE A. Perovskite solar cells go lead free[J]. Joule, 2017, 1(4):659-664. doi: 10.1016/j.joule.2017.09.007 [64] GANOSE A M, SAVORY C N, SCANLON D O. Beyond methylammonium lead iodide:prospects for the emergent field of ns2 containing solar absorbers[J]. Chemical Communications, 2017, 53(1):20-44. doi: 10.1039/C6CC06475B [65] CHAKRABORTY S, XIE W, MATHEWS N, et al.. Rational design:a high-throughput computational screening and experimental validation methodology for lead-free and emergent hybrid perovskites[J]. ACS Energy Letters, 2017, 2(4):837-845. doi: 10.1021/acsenergylett.7b00035 [66] KAMAT P V, BISQUERT J, BURIAK J. Lead-free perovskite solar cells[J]. ACS Energy Letters, 2017, 2(4):904-905. doi: 10.1021/acsenergylett.7b00246 [67] CHEN W, WU Y ZH, YUE Y F, et al.. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers[J]. Science, 2015, 350(6263):944-948. doi: 10.1126/science.aad1015 [68] JEON N J, NOH J H, KIM Y C, et al.. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells[J]. Nature Materials, 2014, 13(9):897-903. doi: 10.1038/nmat4014 [69] SHUKLA S, SHUKLA S, HAUR L J, et al.. Effect of formamidinium/Cesium substitution and PbI2 on the long-term stability of triple-cation perovskites[J]. ChemSusChem, 2017, 10(19):3804-3809. doi: 10.1002/cssc.201701203 [70] EDRI E, KIRMAYER S, KULBAK M, et al.. Chloride inclusion and hole transport material doping to improve methyl ammonium lead bromide perovskite-based high open-circuit voltage solar cells[J]. The Journal of Physical Chemistry Letters, 2014, 5(3):429-433. doi: 10.1021/jz402706q [71] CAO K, LI H, LIU SH SH, et al.. MAPbI3-xBrx mixed halide perovskites for fully printable mesoscopic solar cells with enhanced efficiency and less hysteresis[J]. Nanoscale, 2016, 8(16):8839-8846. doi: 10.1039/C6NR01043A [72] COLELLA S, MOSCONI E, FEDELI P, et al.. MAPbI3-xClx mixed halide perovskite for hybrid solar cells:the role of chloride as dopant on the transport and structural properties[J]. Chemistry of Materials, 2013, 25(22):4613-4618. doi: 10.1021/cm402919x [73] SUAREZ B, GONZALEZ-PEDRO V, RIPOLLES T S, et al.. Recombination study of combined halides(Cl, Br, I) perovskite solar cells[J]. The Journal of Physical Chemistry Letters, 2014, 5(10):1628-1635. doi: 10.1021/jz5006797 [74] EPERON G E, STRANKS S D, MENELAOU C, et al.. Formamidinium lead trihalide:a broadly tunable perovskite for efficient planar heterojunction solar cells[J]. Energy & Environmental Science, 2014, 7(3):982-988. https://pubs.rsc.org/en/content/articlelanding/2014/EE/c3ee43822h#!divAbstract [75] WANG Z W, ZHOU Y Y, PANG SH P, et al.. Additive-modulated evolution of HC(NH2)2PbI3 black polymorph for mesoscopic perovskite solar cells[J]. Chemistry of Materials, 2015, 27(20):7149-7155. doi: 10.1021/acs.chemmater.5b03169 [76] CHOI H, JEONG J, KIM H B, et al.. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells[J]. Nano Energy, 2014, 7:80-85. doi: 10.1016/j.nanoen.2014.04.017 [77] MCMEEKIN D P, SADOUGHI G, REHMAN W, et al.. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells[J]. Science, 2016, 351(6269):151-155. doi: 10.1126/science.aad5845 [78] WANG P Y, ZHANG X W, ZHOU Y Q, et al.. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells[J]. Nature Communications, 2018, 9:2225. doi: 10.1038/s41467-018-04636-4 [79] WANG Y, ZHANG T Y, KAN M, et al.. Bifunctional stabilization of all-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics[J]. Journal of the American Chemical Society, 2018, 140(39):12345-12348. doi: 10.1021/jacs.8b07927 [80] HWANG I, JEONG I, LEE J, et al.. Enhancing stability of perovskite solar cells to moisture by the facile hydrophobic passivation[J]. ACS Applied Materials & Interfaces, 2015, 7(31):17330-17336. https://www.ncbi.nlm.nih.gov/pubmed/26154828 [81] SUPASAI T, RUJISAMPHAN N, ULLRICH K, et al.. Formation of a passivating CH3NH3PbI3/PbI2 interface during moderate heating of CH3NH3PbI3 layers[J]. Applied Physics Letters, 2013, 103(18):183906. doi: 10.1063/1.4826116 [82] WANG L L, MCCLEESE C, KOVALSKY A, et al.. Femtosecond time-resolved transient absorption spectroscopy of CH3NH3PbI3 perovskite films:evidence for passivation effect of PbI2[J]. Journal of the American Chemical Society, 2014, 136(35):12205-12208. doi: 10.1021/ja504632z [83] BU X N, WESTBROOK R J E, LANZETTA L, et al.. Surface passivation of perovskite films via iodide salt coatings for enhanced stability of organic lead halide perovskite solar cells[J]. Solar RRL, 2019, 3(2):1800282. [84] HOU Y, ZHOU Z R, WEN T Y, et al.. Enhanced moisture stability of metal halide perovskite solar cells based on sulfur oleylamine surface modification[J]. Nanoscale Horizons, 2019, 4(1):208-213. doi: 10.1039/C8NH00163D [85] DOMANSKI K, CORREA-BAENA J P, MINE N, et al.. Not all that glitters is gold:metal-migration-induced degradation in perovskite solar cells[J]. ACS Nano, 2016, 10(6):6306-6314. doi: 10.1021/acsnano.6b02613 [86] DA P M, CHA M Y, SUN L, et al.. High-performance perovskite photoanode enabled by Ni passivation and catalysis[J]. Nano Letters, 2015, 15(5):3452-3457. doi: 10.1021/acs.nanolett.5b00788 [87] WANG CH W, YANG SH, CHEN X, et al.. Surface-functionalized perovskite films for stable photoelectrochemical water splitting[J]. Journal of Materials Chemistry A, 2017, 5(3):910-913. doi: 10.1039/C6TA08812K [88] GINTING R T, JEON M K, LEE K J, et al.. Degradation mechanism of planar-perovskite solar cells:correlating evolution of iodine distribution and photocurrent hysteresis[J]. Journal of Materials Chemistry A, 2017, 5(9):4527-4534. doi: 10.1039/C6TA09202K [89] LI Y L, SUN W H, YAN W B, et al.. 50% Sn-based planar perovskite solar cell with power conversion efficiency up to 13.6%[J]. Advanced Energy Materials, 2016, 6(24):1601353. doi: 10.1002/aenm.201601353 [90] WANG ZH K, LI M, YANG Y G, et al.. High efficiency Pb-In binary metal perovskite solar cells[J]. Advanced Materials, 2016, 28(31):6695-6703. doi: 10.1002/adma.201600626 [91] HAO F, STOUMPOS C C, CAO D H, et al.. Lead-free solid-state organic inorganic halide perovskite solar cells[J]. Nature Photonics, 2014, 8(6) 489-494. doi: 10.1038/nphoton.2014.82 [92] LIAO W Q, ZHAO D W, YU Y, et al.. Lead-free inverted planar formamidinium tin triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22%[J]. Advanced Materials, 2016, 28(42):9333-9340. doi: 10.1002/adma.201602992 [93] KUMAR A, BALASUBRAMANIAM K R, KANGSABANIK J, et al.. Crystal structure, stability, and optoelectronic properties of the organic-inorganic wide-band-gap perovskite CH3NH3BaI3:candidate for transparent conductor applicationsapplications[J]. Physical Review B, 2016, 94(18):180105. doi: 10.1103/PhysRevB.94.180105 [94] KRISHNAMOORTHY T, DING H, YAN CH, et al.. Lead-free germanium iodide perovskite materials for photovoltaic applications[J]. Journal of Materials Chemistry A, 2015, 3(47):23829-23832. doi: 10.1039/C5TA05741H [95] MING W M, SHI H L, DU M H. Large dielectric constant, high acceptor density, and deep electron traps in perovskite solar cell material CsGeI3[J]. Journal of Materials Chemistry A, 2016, 4(36):13852-13858. doi: 10.1039/C6TA04685A [96] NOEL N K, STRANKS S D, ABATE A, et al.. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications[J]. Energy & Environmental Science, 2014, 7(9):3061-3068. https://pubs.rsc.org/en/content/articlelanding/2014/EE/C4EE01076K#!divAbstract [97] SHAO SH Y, LIU J, PORTALE G, et al.. Highly reproducible Sn-based hybrid perovskite solar cells with 9% efficiency[J]. Advanced Energy Materials, 2018, 8(4):1702019. doi: 10.1002/aenm.201702019 [98] WANG F, JIANG X Y, CHEN H, et al.. 2D-Quasi-2D-3D hierarchy structure for tin perovskite solar cells with enhanced efficiency and stability[J]. Joule, 2018, 2(12):2732-2743. doi: 10.1016/j.joule.2018.09.012 [99] STOUMPOS C C, FRAZER L, CLARK D J, et al.. Hybrid germanium iodide perovskite semiconductors:active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties[J]. Journal of the American Chemical Society, 2015, 137(21):6804-6819. doi: 10.1021/jacs.5b01025 [100] CHEN M, JU M G, GARCES H F, et al.. Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation[J]. Nature Communications, 2019, 10:16. doi: 10.1038/s41467-018-07951-y [101] YANG D W, LV J, ZHAO X G, et al.. Functionality-directed screening of Pb-free hybrid organic-inorganic perovskites with desired intrinsic photovoltaic functionalities[J]. Chemistry of Materials, 2017, 29(2):524-538. doi: 10.1021/acs.chemmater.6b03221 [102] JIANG Q L, REBOLLAR D, GONG J, et al.. Pseudohalide-induced moisture tolerance in perovskite CH3NH3Pb(SCN)2I thin films[J]. Angewandte Chemie International Edition, 2015, 54(26):7617-7620. doi: 10.1002/anie.201503038 [103] LIANG J, WANG C X, WANG Y R, et al.. All-inorganic perovskite solar cells[J]. Journal of the American Chemical Society, 2016, 138(49):15829-15832. doi: 10.1021/jacs.6b10227 [104] LIANG J, ZHAO P Y, WANG C X, et al.. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability[J]. Journal of the American Chemical Society, 2017, 139(40):14009-14012. doi: 10.1021/jacs.7b07949 [105] LIANG J, LIU J, JIN ZH. All-inorganic halide perovskites for optoelectronics:progress and prospects[J]. Solar RRL, 2017, 1(10):1700086. doi: 10.1002/solr.201700086 [106] DIRIN D N, CHERNIUKH I, YAKUNIN S, et al.. Solution-grown CsPbBr3 perovskite single crystals for photon detection[J]. Chemistry of Materials, 2016, 28(23):8470-8474. doi: 10.1021/acs.chemmater.6b04298 [107] PROTESESCU L, YAKUNIN S, BODNARCHUK M I, et al.. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I):novel optoelectronic materials showing bright emission with wide color gamut[J]. Nano Letters, 2015, 15(6):3692-3696. doi: 10.1021/nl5048779 [108] ZHANG T K, LONG M ZH, YAN K Y, et al.. Crystallinity preservation and ion migration suppression through dual ion exchange strategy for stable mixed perovskite solar cells[J]. Advanced Energy Materials, 2017, 7(15):1700118. doi: 10.1002/aenm.201700118 [109] SLAVNEY A H, HU T, LINDENBERG A M, et al.. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications[J]. Journal of the American Chemical Society, 2016, 138(7):2138-2141. doi: 10.1021/jacs.5b13294 [110] VOLONAKIS G, FILIP M R, HAGHIGHIRAD A A, et al.. Lead-free halide double perovskites via heterovalent substitution of noble metals[J]. The Journal of Physical Chemistry Letters, 2016, 7(7):1254-1259. doi: 10.1021/acs.jpclett.6b00376 [111] MCCLURE E T, BALL M R, WINDL W, et al.. Cs2AgBiX6(X=Br, Cl):new visible light absorbing, lead-free halide perovskite semiconductors[J]. Chemistry of Materials, 2016, 28(5):1348-1354. doi: 10.1021/acs.chemmater.5b04231 [112] FILIP M R, HILLMAN S, HAGHIGHIRAD A A, et al.. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment[J]. The Journal of Physical Chemistry Letters, 2016, 7(13):2579-2585. doi: 10.1021/acs.jpclett.6b01041 [113] ZHAO X G, YANG J H, FU Y H, et al.. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation[J]. Journal of the American Chemical Society, 2017, 139(7):2630-2638. doi: 10.1021/jacs.6b09645 [114] VOLONAKIS G, HAGHIGHIRAD A A, SNAITH H J, et al.. Route to stable lead-free double perovskites with the electronic structure of CH3NH3PbI3:a case for mixed-cation[Cs/CH3NH3/CH(NH2)2]2InBiBr6[J]. The Journal of Physical Chemistry Letters, 2017, 8(16):3917-3924. doi: 10.1021/acs.jpclett.7b01584 [115] QUAN L N, YUAN M J, COMIN R, et al.. Ligand-stabilized reduced-dimensionality perovskites[J]. Journal of the American Chemical Society, 2016, 138(8):2649-2655. doi: 10.1021/jacs.5b11740 [116] TRAN T T, PANELLA J R, CHAMORRO J R, et al.. Designing indirect-direct bandgap transitions in double perovskites[J]. Materials Horizons, 2017, 4(4):688-693. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2e6aed3b09168c262ccfa2c535d66b1b [117] DU K ZH, MENG W W, WANG X M, et al.. Bandgap engineering of lead-free double perovskite Cs2AgBiBr6 through trivalent metal alloying[J]. Angewandte Chemie International Edition, 2017, 56(28):8158-8162. doi: 10.1002/anie.201703970 [118] SLAVNEY A H, LEPPERT L, BARTESAGHI D, et al.. Defect-induced band-edge reconstruction of a bismuth-halide double perovskite for visible-light absorption[J]. Journal of the American Chemical Society, 2017, 139(14):5015-5018. doi: 10.1021/jacs.7b01629 [119] GREUL E, PETRUS M L, BINEK A, et al.. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications[J]. Journal of Materials Chemistry A, 2017, 5(37):19972-19981. doi: 10.1039/C7TA06816F [120] NING W H, WANG F, WU B, et al.. Long electron-hole diffusion length in high-quality lead-free double perovskite films[J]. Advanced Materials, 2018, 30(20):1706246. doi: 10.1002/adma.201706246 [121] WU C C, ZHANG Q H, LIU Y, et al.. The dawn of lead-free perovskite solar cell:highly stable double perovskite Cs2AgBiBr6 film[J]. Advanced Science, 2018, 5(3):1700759. doi: 10.1002/advs.201700759 [122] WEI F X, DENG Z Y, SUN SH J, et al.. Synthesis and properties of a lead-free hybrid double perovskite:(CH3NH3)2AgBiBr6[J]. Chemistry of Materials, 2017, 29(3):1089-1094. doi: 10.1021/acs.chemmater.6b03944 [123] CHENG P F, WU T, LI Y J, et al.. Combining theory and experiment in the design of a lead-free ((CH3NH3)2AgBiI6) double perovskite[J]. New Journal of Chemistry, 2017, 41(18):9598-9601. doi: 10.1039/C7NJ02365K [124] ZHAO X G, YANG D W, SUN Y H, et al.. Cu-In halide perovskite solar absorbers[J]. Journal of the American Chemical Society, 2017, 139(19):6718-6725. doi: 10.1021/jacs.7b02120 [125] VOLONAKIS G, HAGHIGHIRAD A A, MILOT R L, et al.. Cs2InAgCl6:a new lead-free halide double perovskite with direct band gap[J]. The Journal of Physical Chemistry Letters, 2017, 8(4):772-778. doi: 10.1021/acs.jpclett.6b02682 [126] MENG W W, WANG X M, XIAO Z W, et al.. Parity-forbidden transitions and their impact on the optical absorption properties of lead-free metal halide perovskites and double perovskites[J]. The Journal of Physical Chemistry Letters, 2017, 8(13):2999-3007. doi: 10.1021/acs.jpclett.7b01042 [127] ZHOU J, XIA ZH G, MOLOKEEV M S, et al.. Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6[J]. Journal of Materials Chemistry A, 2017, 5(29):15031-15037. doi: 10.1039/C7TA04690A [128] LUO J J, WANG X M, LI SH R, et al.. Efficient and stable emission of warm-white light from lead-free halide double perovskites[J]. Nature, 2018, 563(7732):541-545. doi: 10.1038/s41586-018-0691-0 [129] K N N, NAG A. Synthesis and luminescence of Mn-doped Cs2AgInCl6 double perovskites[J]. Chemical Communications, 2018, 54(41):5205-5208. doi: 10.1039/C8CC01982G [130] XIAO Z W, DU K ZH, MENG W W, et al.. Chemical origin of the stability difference between copper(Ⅰ)-and silver(Ⅰ)-based halide double perovskites[J]. Angewandte Chemie International Edition, 2017, 129(40):12275-12279. https://www.ncbi.nlm.nih.gov/pubmed/28755410 [131] DENG Z Y, WEI F X, SUN SH J, et al.. Exploring the properties of lead-free hybrid double perovskites using a combined computational-experimental approach[J]. Journal of Materials Chemistry A, 2016, 4(31):12025-12029. doi: 10.1039/C6TA05817E [132] XIAO Z W, ZHOU Y Y, HOSONO H, et al.. Bandgap optimization of perovskite semiconductors for photovoltaic applications[J]. Chemistry A European Journal, 2018, 24(10):2305-2316. doi: 10.1002/chem.201705031 [133] CREUTZ S E, CRITES E N, DE SIENA M C, et al.. Colloidal nanocrystals of lead-free double-perovskite(Elpasolite) semiconductors:synthesis and anion exchange to access new materials[J]. Nano Letters, 2018, 18(2):1118-1123. doi: 10.1021/acs.nanolett.7b04659 [134] DENG W, DENG Z Y, HE J W, et al.. Synthesis of Cs2AgSbCl6 and improved optoelectronic properties of Cs2AgSbCl6/TiO2 heterostructure driven by the interface effect for lead-free double perovskites solar cells[J]. Applied Physics Letters, 2017, 111(15):151602. doi: 10.1063/1.4999192 [135] LUO J J, LI SH R, WU H D, et al.. Cs2AgInCl6 double perovskite single crystals:parity forbidden transitions and their application for sensitive and fast uv photodetectors[J]. ACS Photonics, 2018, 5(2):398-405. doi: 10.1021/acsphotonics.7b00837 [136] XIAO Z W, ZHOU Y Y, HOSONO H, et al.. Intrinsic defects in a photovoltaic perovskite variant Cs2SnI6[J]. Physical Chemistry Chemical Physics, 2015, 17(29):18900-18903. doi: 10.1039/C5CP03102H [137] XIAO Z W, LEI H CH, ZHANG X, et al.. Ligand-hole in[SnI6] unit and origin of band gap in photovoltaic perovskite variant Cs2SnI6[J]. Bulletin of the Chemical Society of Japan, 2015, 88(9):1250-1255. doi: 10.1246/bcsj.20150110 [138] LEE B, STOUMPOS C C, ZHOU N J, et al.. Air-stable molecular semiconducting iodosalts for solar cell applications:Cs2SnI6 as a hole conductor[J]. Journal of the American Chemical Society, 2014, 136(43):15379-15385. doi: 10.1021/ja508464w [139] SAPAROV B, SUN J P, MENG W W, et al.. Thin-film deposition and characterization of a Sn-deficient perovskite derivative Cs2SnI6[J]. Chemistry of Materials, 2016, 28(7):2315-2322. doi: 10.1021/acs.chemmater.6b00433 [140] SAKAI N, HAGHIGHIRAD A A, FILIP M R, et al.. Solution-processed cesium hexabromopalladate(Ⅳ), Cs2PdBr6, for optoelectronic applications[J]. Journal of the American Chemical Society, 2017, 139(17):6030-6033. doi: 10.1021/jacs.6b13258 [141] MAUGHAN A E, GANOSE A M, BORDELON M M, et al.. Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6[J]. Journal of the American Chemical Society, 2016, 138(27):8453-8464. doi: 10.1021/jacs.6b03207 [142] DONALDSON L. Lead-free perovskites improve solar energy[J]. Materials Today, 2018, 21(5):460. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cbf06fbedf4b145e44a8d2182c409106 [143] TSAI H, NIE W Y, BLANCON J C, et al.. High-efficiency two-dimensional Ruddlesden Popper perovskite solar cells[J]. Nature, 2016, 536(7616):312-316. doi: 10.1038/nature18306 [144] FRACCAROLLO A, CANTATORE V, BOSCHETTO G, et al.. Ab initio modeling of 2D layered organohalide lead perovskites[J]. The Journal of Chemical Physics, 2016, 144(16):164701. doi: 10.1063/1.4947305 [145] BLANCON J C, TSAI H, NIE W, et al.. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites[J]. Science, 2017, 355(6331):1288-1292. doi: 10.1126/science.aal4211 [146] LIAO J F, RAO H SH, CHEN B X, et al.. Dimension engineering on cesium lead iodide for efficient and stable perovskite solar cells[J]. Journal of Materials Chemistry A, 2017, 5(5):2066-2072. doi: 10.1039/C6TA09582H [147] HAMAGUCHI R, YOSHIZAWA-FUJITA M, MIYASAKA T, et al.. Formamidine and cesium-based quasi-two-dimensional perovskites as photovoltaic absorbers[J]. Chemical Communications, 2017, 53(31):4366-4369. doi: 10.1039/C7CC00921F [148] LIU Y Y, XIAO H, GODDARD Ⅲ W A. Two-dimensional halide perovskites:tuning electronic activities of defects[J]. Nano Letters, 2016, 16(5):3335-3340. doi: 10.1021/acs.nanolett.6b00964 [149] MA L, DAI J, ZENG X CH. Two-dimensional single-layer organic inorganic hybrid perovskite semiconductors[J]. Advanced Energy Materials, 2017, 7(7):1601731. doi: 10.1002/aenm.201601731 [150] ZHANG L, LIANG W ZH. How the structures and properties of two-dimensional layered perovskites MAPbI3 and CsPbI3 vary with the number of layers[J]. The Journal of Physical Chemistry Letters, 2017, 8(7):1517-1523. doi: 10.1021/acs.jpclett.6b03005 [151] SHANG Q Y, WANG Y N, ZHONG Y G, et al.. Unveiling structurally engineered carrier dynamics in hybrid quasi-two-dimensional perovskite thin films toward controllable emission[J]. The Journal of Physical Chemistry Letters, 2017, 8(18):4431-4438. doi: 10.1021/acs.jpclett.7b01857 [152] LIU J X, LENG J, WU K F, et al.. Observation of internal photoinduced electron and hole separation in hybrid two-dimentional perovskite films[J]. Journal of the American Chemical Society, 2017, 139(4):1432-1435. doi: 10.1021/jacs.6b12581 [153] LIAO Y Q, LIU H F, ZHOU W J, et al.. Highly oriented low-dimensional tin halide perovskites with enhanced stability and photovoltaic performance[J]. Journal of the American Chemical Society, 2017, 139(19):6693-6699. doi: 10.1021/jacs.7b01815 [154] CAO D H, STOUMPOS C C, YOKOYAMA T, et al.. Thin films and solar cells based on semiconducting two-dimensional Ruddlesden-Popper (CH3(CH2)3NH3)2(CH3NH3)n-1SnnI3n+1 Perovskites[J]. ACS Energy Letters, 2017, 2(5):982-990. doi: 10.1021/acsenergylett.7b00202 [155] SAPAROV B, HONG F, SUN J P, et al.. Thin-film preparation and characterization of Cs3Sb2I9:a lead-free layered perovskite semiconductor[J]. Chemistry of Materials, 2015, 27(16):5622-5632. doi: 10.1021/acs.chemmater.5b01989 [156] PARK B W, PHILIPPE B, ZHANG X L, et al.. Bismuth based hybrid perovskites A3Bi2I9(A:Methylammonium or Cesium) for solar cell application[J]. Advanced Materials, 2015, 27(43):6806-6813. doi: 10.1002/adma.201501978 [157] OLDAG T, AUSSIEKER T, KELLER H L, et al.. Solvothermale synthese und bestimmung der kristallstrukturen von AgBiI4 und Ag3BiI6[J]. Zeitschrift für Anorganische und Allgemeine Chemie, 2005, 631(4):677-682. doi: 10.1002/zaac.200400508 [158] ZHANG ZH, LI X W, XIA X H, et al.. High-quality (CH3NH3)3Bi2I9 film-based solar cells:pushing efficiency up to 1.64%[J]. The Journal of Physical Chemistry Letters, 2017, 8(17):4300-4307. doi: 10.1021/acs.jpclett.7b01952 [159] BAI F, HU Y H, HU Y Q, et al.. Lead-free, air-stable ultrathin Cs3Bi2I9 perovskite nanosheets for solar cells[J]. Solar Energy Materials and Solar Cells, 2018, 184:15-21. doi: 10.1016/j.solmat.2018.04.032 [160] PAL J, MANNA S, MONDAL A, et al.. Colloidal synthesis and photophysics of M3Sb2I9(M=Cs and Rb) nanocrystals:lead-free perovskites[J]. Angewandte Chemie International Edition, 2017, 56(45):14187-14191. doi: 10.1002/anie.201709040 [161] JIANG F Y, YANG D W, JIANG Y Y, et al.. Chlorine-incorporation-induced formation of the layered phase for antimony-based lead-free perovskite solar cells[J]. Journal of the American Chemical Society, 2018, 140(3):1019-1027. doi: 10.1021/jacs.7b10739 [162] JOHANSSON M B, ZHU H M, JOHANSSON E M J. Extended photo-conversion spectrum in low-toxic bismuth halide perovskite solar cells[J]. The Journal of Physical Chemistry Letters, 2016, 7(17):3467-3471. doi: 10.1021/acs.jpclett.6b01452 [163] SANSOM H C, WHITEHEAD G F S, DYER M S, et al.. AgBiI4 as a lead-free solar absorber with potential application in photovoltaics[J]. Chemistry of Materials, 2017, 29(4):1538-1549. doi: 10.1021/acs.chemmater.6b04135 [164] XIAO Z W, MENG W W, MITZI D B, et al.. Crystal structure of AgBi2I7 thin films[J]. The Journal of Physical Chemistry Letters, 2016, 7(19):3903-3907. doi: 10.1021/acs.jpclett.6b01834 [165] PAI N, LU J F, GENGENBACH T R, et al.. Silver bismuth sulfoiodide solar cells:tuning optoelectronic properties by sulfide modification for enhanced photovoltaic performance[J]. Advanced Energy Materials, 2019, 9(5):1803396. [166] MASHADIEVA L F, ALIEV Z S, SHEVELKOV A V, et al.. Experimental investigation of the Ag-Bi-I ternary system and thermodynamic properties of the ternary phases[J]. Journal of Alloys and Compounds, 2013, 551:512-520. doi: 10.1016/j.jallcom.2012.11.033 [167] JU M G, CHEN M, ZHOU Y Y, et al.. Earth-abundant nontoxic titanium(Ⅳ)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications[J]. ACS Energy Letters, 2018, 3(2):297-304. doi: 10.1021/acsenergylett.7b01167 -






 下载:
下载: