Investigation of self-doping in perovskites with vacancy defects based on first principles
-
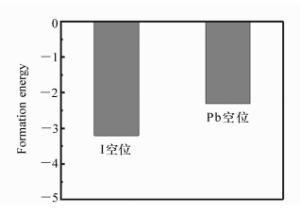
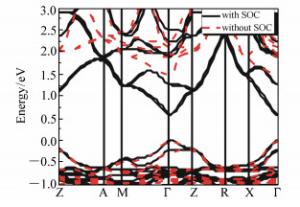
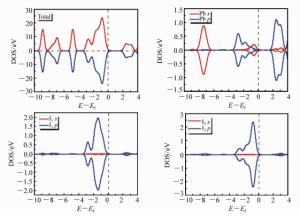
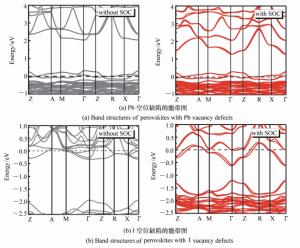
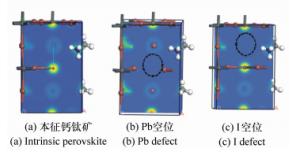
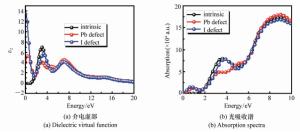
摘要: 为了获得优异的钙钛矿材料,本文系统地研究有机-无机杂化钙钛矿材料(CH3NH3PbI3)的电子结构和光学特性,同时探究了空位缺陷对其光学性质的影响。首先,采用Materials Studio软件构建本征钙钛矿材料的电子结构,并基于广义梯度近似的方法(GGA)和Perdew-Burker-Ernzerhof(PBE)泛函,优化其电子结构并计算本征钙钛矿材料的电学和光学特性。通过采用范德华力修正,解决了密度泛函理论低估带隙的问题,得到准确的带隙。其次,研究不同的空位缺陷(Pb空位和I空位缺陷)对钙钛矿材料的电子结构的影响,并计算其能带、态密度和光学性质。最后通过对比本征钙钛矿材料和空位缺陷的钙钛矿材料特性,从微观机理研究空位缺陷对其光学性质的影响。结果表明:本征钙钛矿材料带隙为1.52 eV,这与实验测得的带隙值基本吻合;同时研究发现Pb空位缺陷会导致钙钛矿呈偏P型材料;I空位缺陷会导致钙钛矿呈偏N型材料。空位缺陷能够有效地改变钙钛矿材料的介电函数和光吸收谱,对于钙钛矿材料的研究及在光电器件领域的应用具有重要的理论价值。Abstract: In order to obtain excellent perovskite materials, we systematically investigate the structural, electronic and optical properties of perovskites and the influence of vacancy defects on their optical properties. First, we explore the structural properties of intrinsic perovskites via Materials Studio and use the Generalized Gradient Approximation(GGA) with the Perdew-Burke-Ernzerhof(PBE) function to optimize the crystal structure and calculate the electronic or optical properties. To obtain accurate bandgap, we use Density Functional Theory-Vander Waals Force(DFT-VDW) correlations to explain the underestimated bandgap. The investigations of the different vacancy defects in perovskites are then carried out and the band structure, density of states and optical properties are measured. Finally, the properties of self-doped perovskites with vacancy defects are further investigated with comparison to the properties of intrinsic perovskites. Our results indicate that the obtained bandgap of intrinsic perovskites was 1.52 eV, which is consistent with the experimental value. The perovskites with Pb vacancy defects belong to P-type materials and those with I vacancy defects belong to N-type materials. The dielectric virtual function and absorption spectrum exhibit substantial change, which has important theoretical value for the physical properties of perovskites and their applications in the fields of optoelectronic devices.
-
Key words:
- perovskite material /
- vacancy defects /
- first principle /
- photoelectric device
-
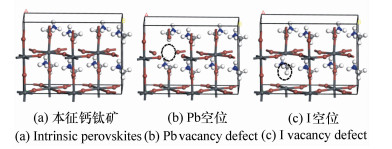
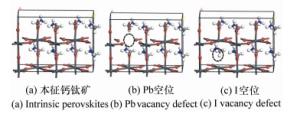
表 1 本征钙钛矿和空位缺陷钙钛矿的晶格常数、键长和键角
Table 1. Lattice constants, bond length and octahedral tilting angles(θ) of intrinsic perovskites and perovskites with vacancy defects
a b c Pb-I θ 本征钙钛矿理论值 8.879 8.929 12.828 3.16~3.28 23.0~27.9 本征钙钛矿实验值 8.91 8.91 12.725 3.15~3.24 23.2~27.9 Pb空位缺陷 8.62 8.65 12.91 3.02~3.15 24.3~28.6 I空位缺陷 8.71 8.79 12.97 3.2~3.32 26.8~29.7 -
[1] LEE M M, TEUSCHER J, MIYASAKA T, et al.. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites[J]. Science, 2012, 338(6107):643-647. doi: 10.1126/science.1228604 [2] STRANKS S D, SNAITH H J. Metal-halide perovskites for photovoltaic and light-emitting devices[J]. Nature Nanotechnology, 2015, 10(5):391-402. doi: 10.1038/nnano.2015.90 [3] WANG J P, WANG N N, JIN Y ZH, et al.. Interfacial control toward efficient and low-voltage perovskite light-emitting diodes[J]. Advanced Materials, 2015, 27(14):2311-2316. doi: 10.1002/adma.201405217 [4] LI F, MA CH, WANG H, et al.. Ambipolar solution-processed hybrid perovskite phototransistors[J]. Nature Communications, 2015, 6:8238. doi: 10.1038/ncomms9238 [5] CHIN X Y, CORTECCHIA D, YIN J, et al.. Lead iodide perovskite light-emitting field-effect transistor[J]. Nature Communications, 2015, 6:7383. doi: 10.1038/ncomms8383 [6] ZHU H M, Fu Y P, MENG F, et al.. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors[J]. Nature Materials, 2015, 14(6):636-642. doi: 10.1038/nmat4271 [7] YOO E J, LYU M, YUN J H, et al.. Resistive switching behavior in organic inorganic hybrid CH3NH3PbI3-xClx perovskite for resistive random access memory devices[J]. Advanced Materials, 2015, 27(40):6170-6175. doi: 10.1002/adma.201502889 [8] KOJIMA A, TESHIMA K, SHIRAI Y, et al.. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells[J]. Journal of the American Chemical Society, 2009, 131(17):6050-6051. doi: 10.1021/ja809598r [9] National Renewable Energy Laboratory(NREL). Research cell efficiency records[EB/OL].[2019-01-10]. https://www.nrel.gov/pv/assets/images/efficiencychart.png. [10] ZHOU Y Y, GAME O S, PANG SH P, et al.. Microstructures of organometal trihalide perovskites for solar cells:their evolution from solutions and characterization[J]. The Journal of Physical Chemistry Letters, 2015, 6(23):4827-4839. doi: 10.1021/acs.jpclett.5b01843 [11] ZHENG L L, ZHANG D F, MA Y ZH, et al.. Morphology control of the perovskite films for efficient solar cells[J]. Dalton Transactions, 2015, 44(23):10582-10593. doi: 10.1039/C4DT03869J [12] MEI A Y, LI X, LIU L F, et al.. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability[J]. Science, 2014, 345(6194):295-298. doi: 10.1126/science.1254763 [13] DOCAMPO P, BALL J M, DARWICH M, et al.. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates[J]. Nature Communications, 2013, 4:2761. doi: 10.1038/ncomms3761 [14] ROLDÁN-CARMONA C, MALINKIEWICZ O, SORIANO A, et al.. Flexible high efficiency perovskite solar cells[J]. Energy & Environmental Science, 2014, 7(3):994-997. http://cn.bing.com/academic/profile?id=54837badce92fa9ac689833770abbb78&encoded=0&v=paper_preview&mkt=zh-cn [15] DYMSHITS A, ROTEM A, ETGAR L, et al.. High voltage in hole conductor free organometal halide perovskite solar cells[J]. Journal of Materials Chemistry A, 2014, 2(48):20776-20781. doi: 10.1039/C4TA05613B [16] HEO J H, SONG D H, IM S H. Planar CH3NH3PbBr3 hybrid solar cells with 10.4% power conversion efficiency, fabricated by controlled crystallization in the spin-coating process[J]. Advanced Materials, 2014, 26(48):8179-8183. doi: 10.1002/adma.201403140 [17] LIANG P W, CHUEH C C, XIN X K, et al.. High-performance planar-heterojunction solar cells based on ternary halide large-band-gap perovskites[J]. Advanced Energy Materials, 2015, 5(1):1400960. doi: 10.1002/aenm.201400960 [18] DIMESSO L, DIMAMAY M, HAMBURGER M, et al.. Properties of CH3NH3PbX3(X=I, Br, Cl) powders as precursors for organic/inorganic solar cells[J]. Chemistry of Materials, 2014, 26(23):6762-6770. doi: 10.1021/cm503240k [19] NAVAS J, SÁNCHEZ-CORONILLA A, GALLARDO J J, et al.. New insights into organic-inorganic hybrid perovskite CH3NH3PbI3 nanoparticles. An experimental and theoretical study of doping in Pb2+ sites with Sn2+, Sr2+, Cd2+ and Ca2+[J]. Nanoscale, 2015, 7(14):6216-6229. doi: 10.1039/C5NR00041F [20] FENG H J, PAUDEL T R, TSYMBAL E Y, et al.. Tunable optical properties and charge separation in CH3NH3SnxPb1-xI3/TiO2-based planar perovskites cells[J]. Journal of the American Chemical Society, 2015, 137(25):8227-8236. doi: 10.1021/jacs.5b04015 [21] ABDELHADY A L, SAIDAMINOV M I, MURALI B, et al.. Heterovalent dopant incorporation for bandgap and type engineering of perovskite crystals[J]. The Journal of Physical Chemistry Letters, 2016, 7(2):295-301. doi: 10.1021/acs.jpclett.5b02681 [22] KAZIM S, NAZEERUDDIN M K, GRÄTZEL M, et al.. Perovskite as light harvester:a game changer in photovoltaics[J]. Angewandte Chemie International Edition, 2014, 53(11):2812-2824. doi: 10.1002/anie.201308719 [23] CHANG J J, LIN ZH H, ZHU H, et al.. Enhancing the photovoltaic performance of planar heterojunction perovskite solar cells by doping the perovskite layer with alkali metal ions[J]. Journal of Materials Chemistry A, 2016, 4(42):16546-16552. doi: 10.1039/C6TA06851K [24] WANG ZH K, LI M, YANG Y G, et al.. High efficiency Pb-In binary metal perovskite solar cells[J]. Advanced Materials, 2016, 28(31):6695-6703. doi: 10.1002/adma.201600626 [25] CAO D H, STOUMPOS C C, MALLIAKAS C D, et al.. Remnant PbI2, an unforeseen necessity in high-efficiency hybrid perovskite-based solar cells?[J]. APL Materials, 2014, 2(9):091101. doi: 10.1063/1.4895038 [26] LEE Y H, LUO J SH, HUMPHRY-BAKER R, et al.. Unraveling the reasons for efficiency loss in perovskite solar cells[J]. Advanced Functional Materials, 2015, 25(25):3925-3933. doi: 10.1002/adfm.201501024 [27] KIM J, LEE S H, LEE J H, et al.. The role of intrinsic defects in methylammonium lead iodide perovskite[J]. The Journal of Physical Chemistry Letters, 2014, 5(8):1312-1317. doi: 10.1021/jz500370k -





 下载:
下载: