Solutions to inhomogeneous and unstable illumination in biological photoacoustic tomography
-
摘要: 在生物组织光声层析成像(Photoacoustic Tomography, PAT)算法中,为了简化问题,通常假设在均匀和稳定照明的理想情况下,重建组织的初始声压分布图、光吸收能量分布图和光学特性参数分布图。但在实际应用中,当光在生物组织中传播时,会出现光衰减和光通量分布不均匀的情况,导致重建精度下降。本文对非理想条件下用于补偿由不均匀和不稳定照明所致PAT成像误差的主要方法进行归纳和总结,讨论不同方法的优势和不足。Abstract: In biological Photoacoustic Tomography (PAT), the images of initial pressure, optical deposition and optical properties are usually reconstructed from acoustic measurements based on an ideal assumption of uniform and stable illumination for simplicity. However, in practical applications, optical attenuation and inhomogeneous distribution of light fluence in tissues may occur after the imaging target is illuminated by short laser pulses, which results in inaccurate image reconstruction and reduced image quality. This paper summarizes current methods for reducing errors caused by inhomogeneous and unstable illumination in PAT under non-ideal conditions and discusses the advantages and limits of these methods.
-
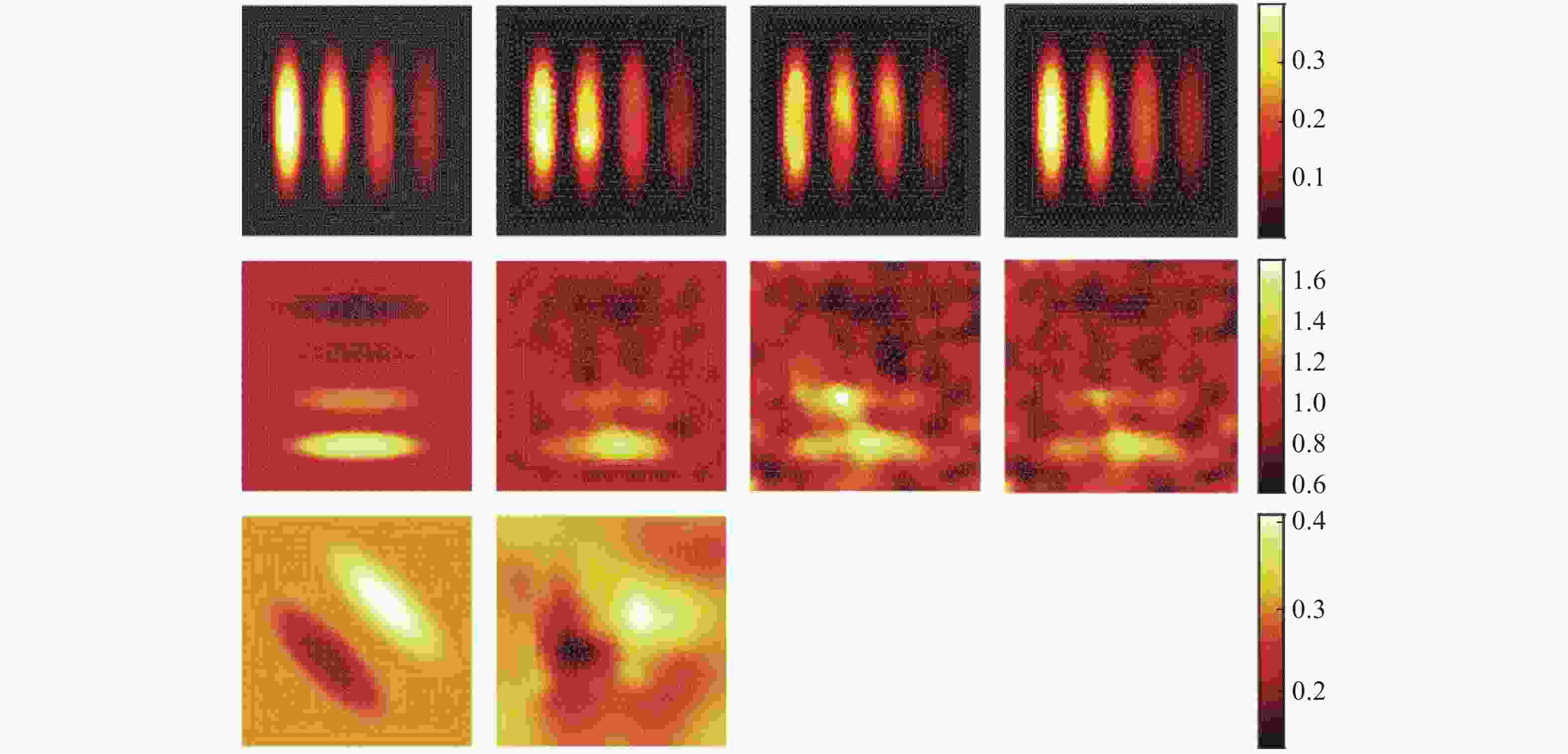
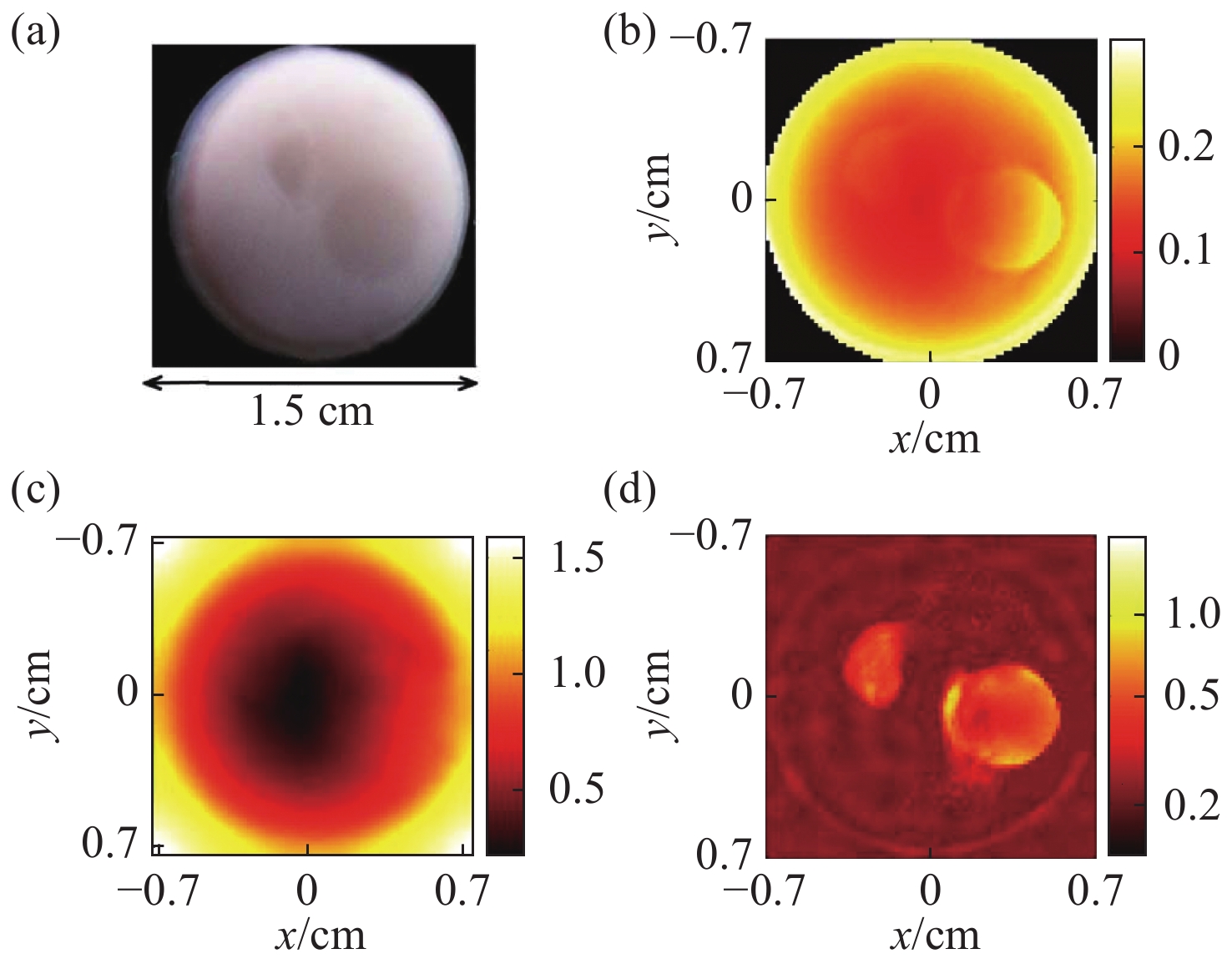
图 1 采用稀疏分解法的PAT图像重建结果[15]。 (a)仿体的几何结构图;(b)光吸收能量分布图;(c)光通量分布图;(d)光吸收系数分布图
Figure 1. Results of PAT image reconstruction by using the sparse decomposition algorithm[15]. (a) Geometry of the phantom to be imaged; (b) optical absorption distribution; (c) light fluence distribution; (d) optical absorption coefficient
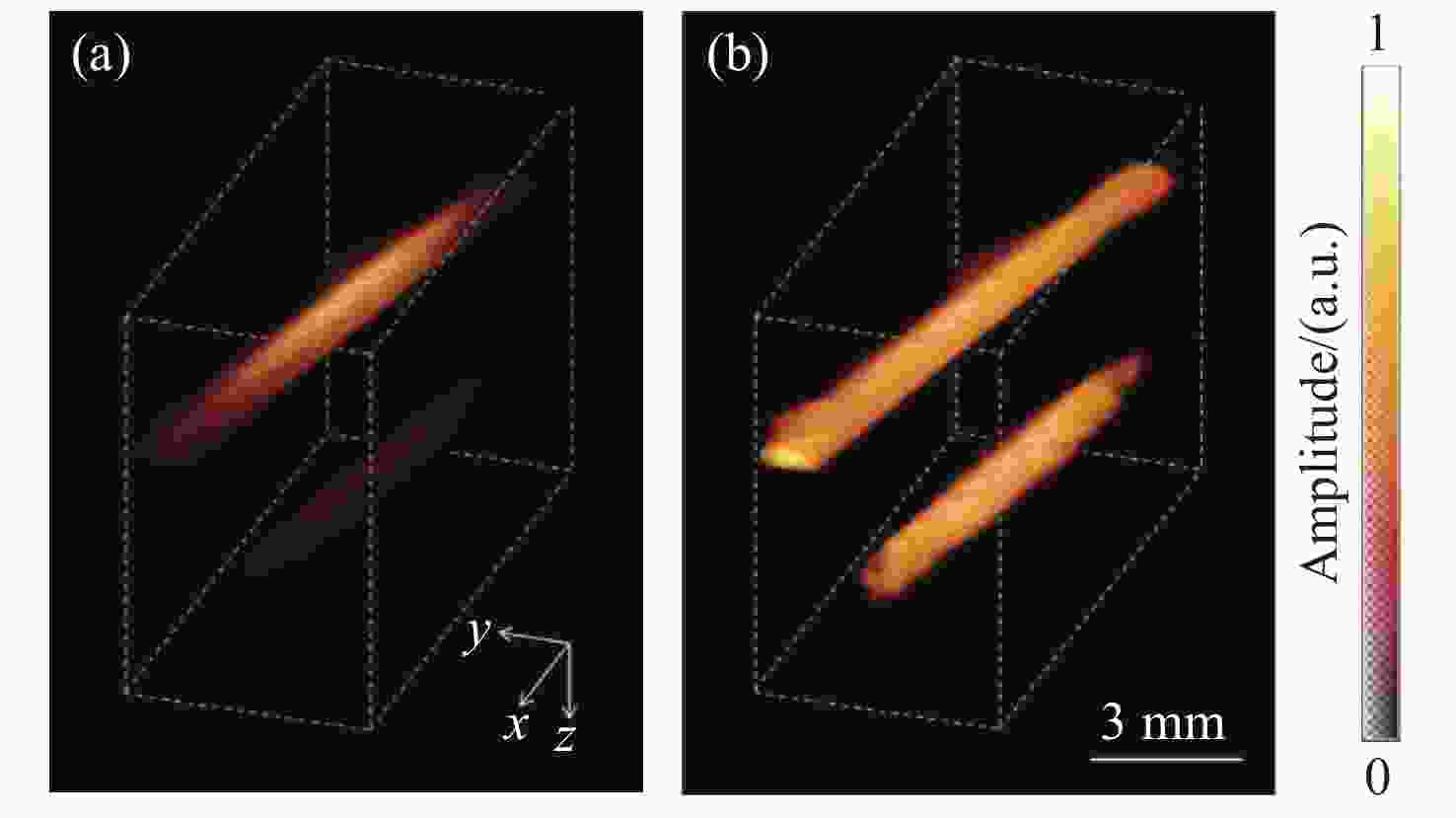
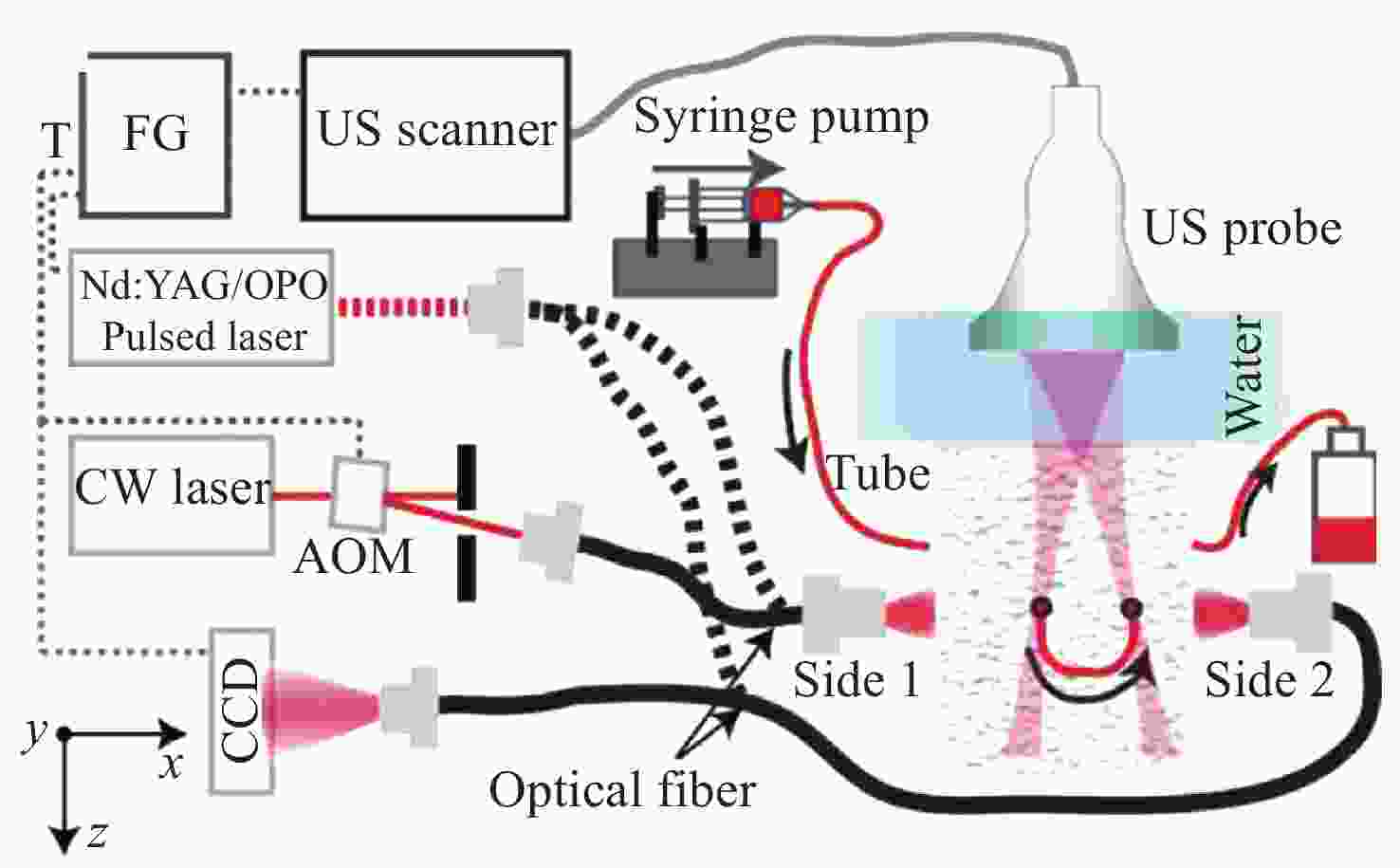
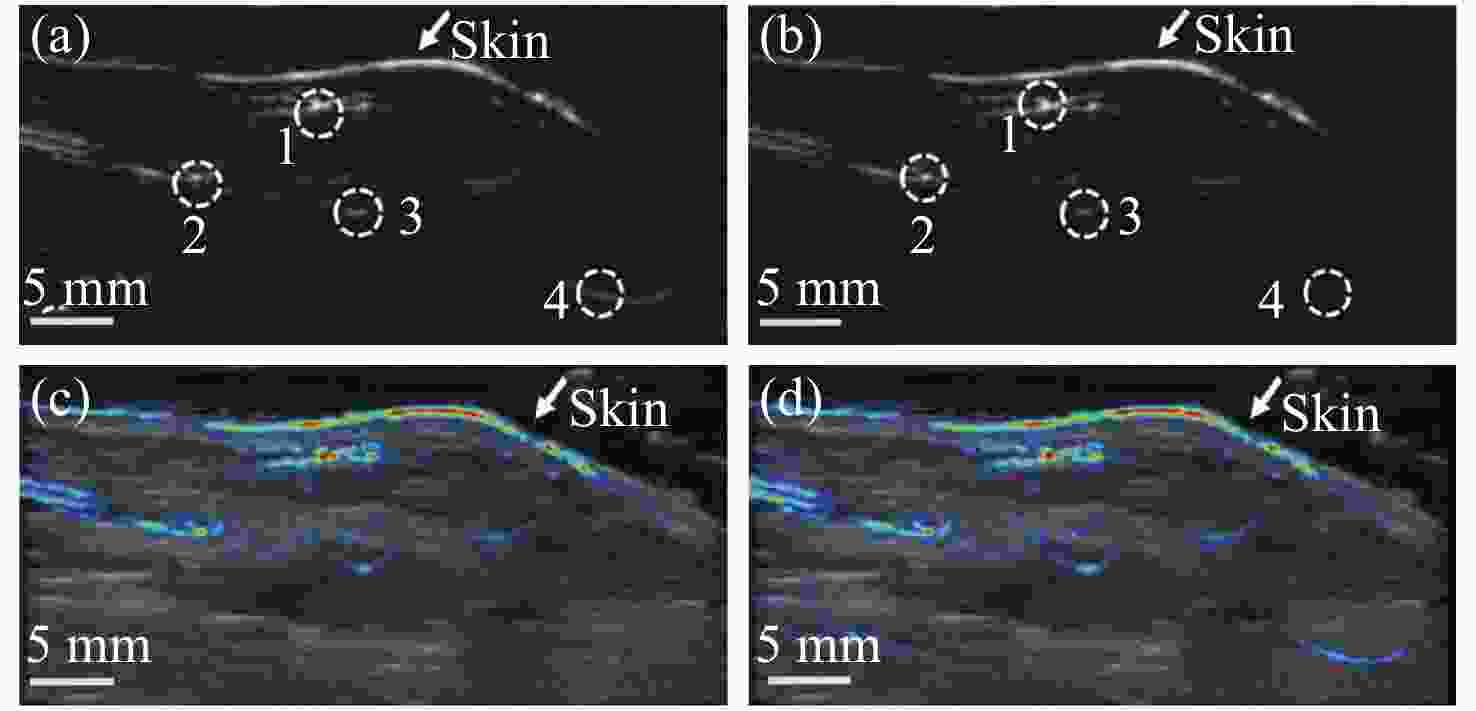
图 4 采用PA-AO光谱组合法得到的光声图像[20]。(a) λ=755 nm时从side1(左图)和side2(右图)照射介质得到的光声图像;(b) λ=780 nm时从side1(左图)和side2(右图)照射介质得到的光声图像;(c) λ=755 nm(左图)和λ=780 nm(右图)时补偿光通量后的光声图像
Figure 4. PA images obtained by using PA-AO spectral combination method[20]. (a) PA images by exciting the medium from side 1 (Left) and side 2 (Right) when λ=755 nm; (b) PA images by exciting the medium from side 1 (Left) and side 2 (Right) when λ=780 nm; (c) PA images of λ=755 nm (Left) and λ=780 nm (Right) after light fluence compensation. Reprinted with permission from © The Optical Society.
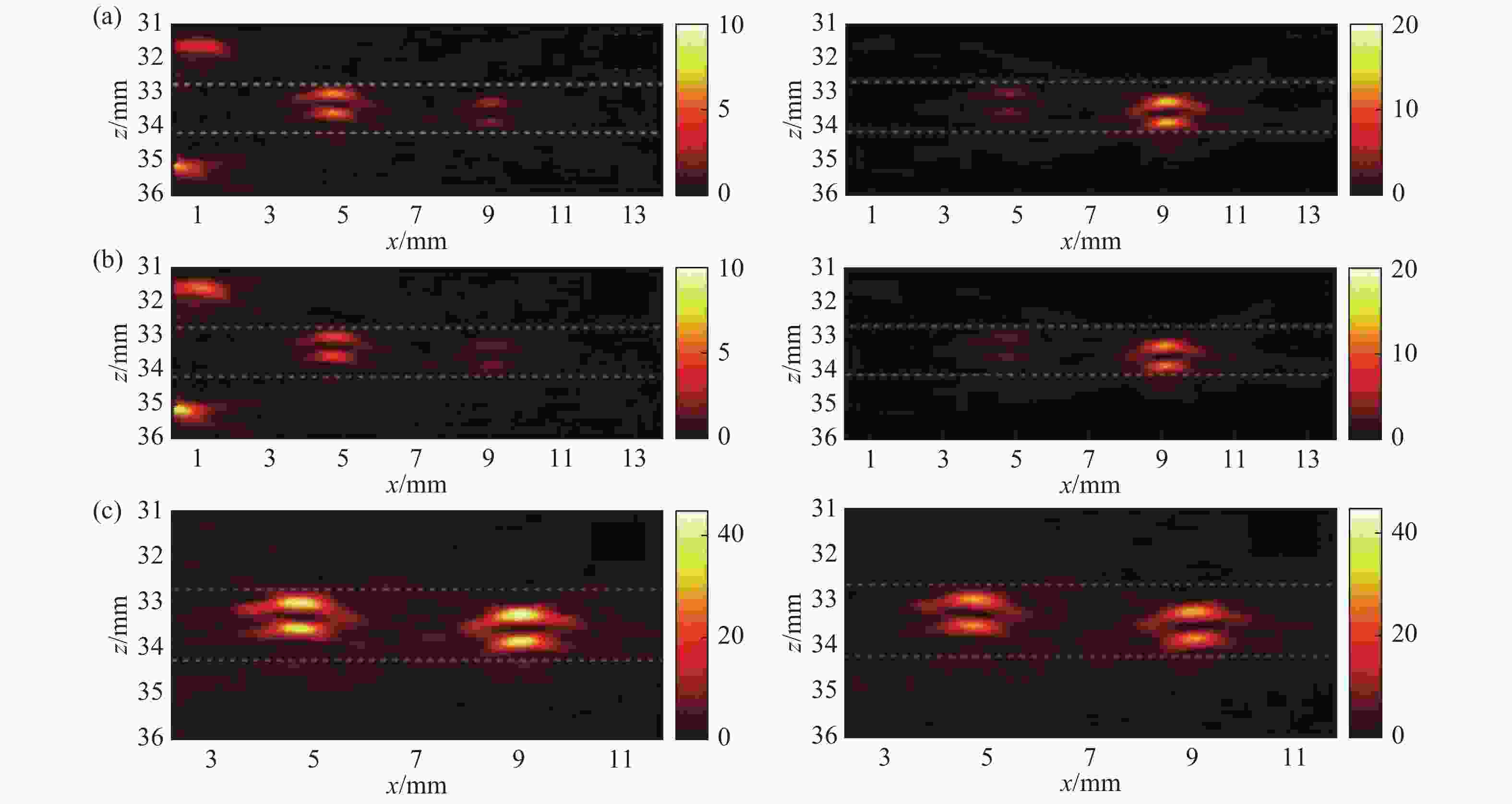
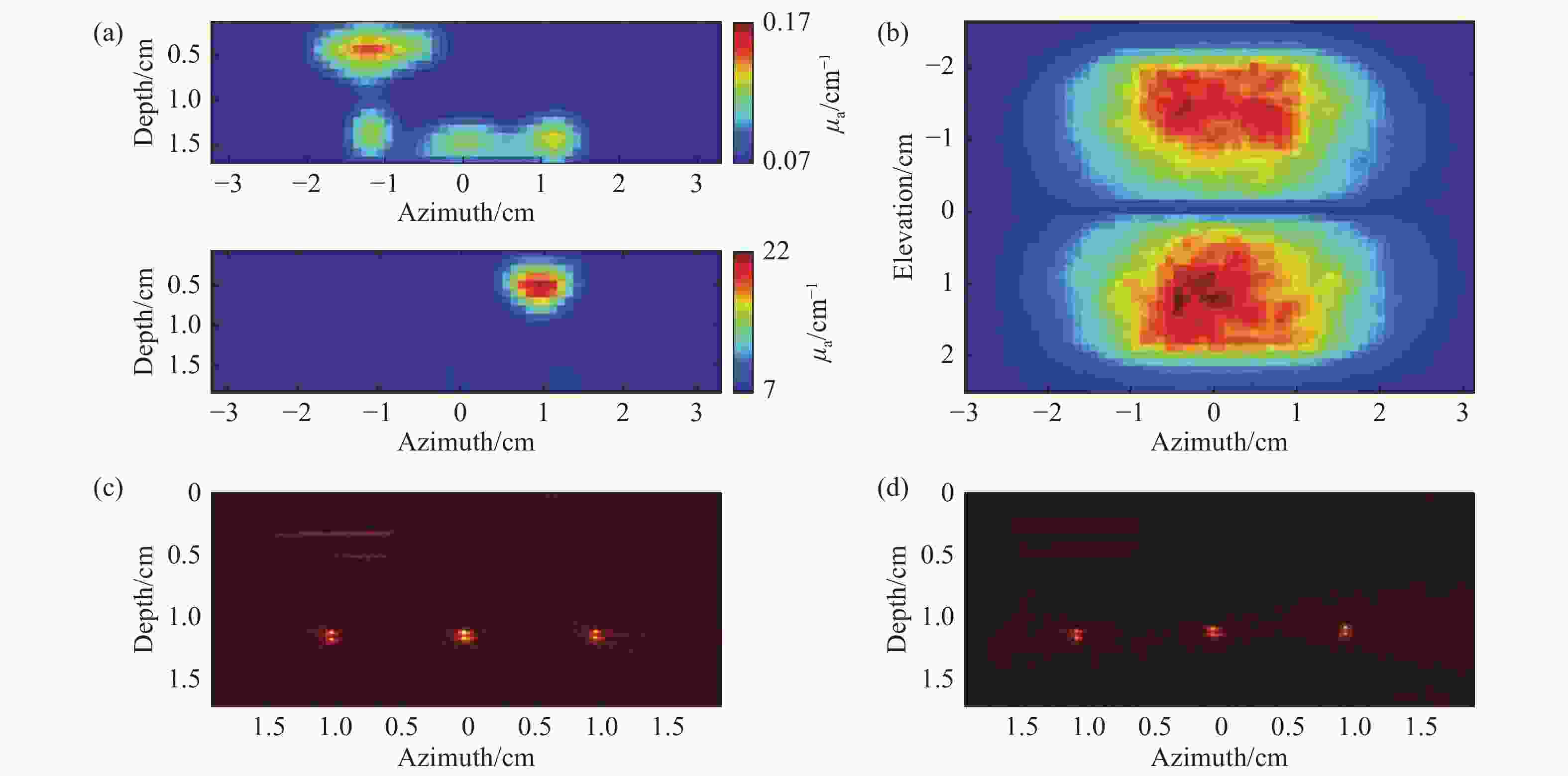
图 9 PAT-DOT的成像结果[28]。 (a) DOT测量的光吸收系数和散射系数分布图;(b)组织表面的光通量分布图;(c)补偿光通量之前的PAT图像;(d)补偿光通量之后的PAT图像
Figure 9. Results of the PAT-DOT method[28]. (a) The distributions of optical absorption coefficient and scattering coefficient measured by DOT; (b) light fluence on the phantom surface; PAT images (c) before and (d) after compensating for light fluence
表 1 补偿PAT光通量变化的主要方法对比
Table 1. Comparison of main methods of compensating for variations in light fluence in PAT
方法 优点 缺点 适用范围 稀疏分解法 无需组织光学特性的先验知识,
不必求解光辐射传输方程,建模
误差不累积在图像重建结果中不适用于光吸收系数缓慢
变化的目标,非均匀边界
光照可能引入误差光照非均匀性高、量化精度高
以及稳定性要求高的静态
目标的成像基于成像模型
的方法减少低光通量区域的噪声和伪影,
减少有限角度测量和声速不均匀
分布所致重建误差噪声抑制效果差,需要较大
内存,很难应用于高分辨率、
大规模、实时图像重建任意光照条件、平面/圆柱/
球面测量几何、静态物体的
小规模成像基于荧光蛋白
的方法光学对比度高,分辨率高,可校正
运动区域的光通量,可在不同
波长下校正光通量可逆蛋白种类有限,波长依赖性 不同入射光波长、静态/
动态目标的成像光声-声光
光谱组合法无需组织光学特性的先验知识,
不依赖于光传输模型的假设波长依赖性,忽略局部
散射变化和超声标记体积
的影响不同入射光波长、静态目标
的成像光声-超声双模态
成像融合法减少由于反向散射和漫反射引起
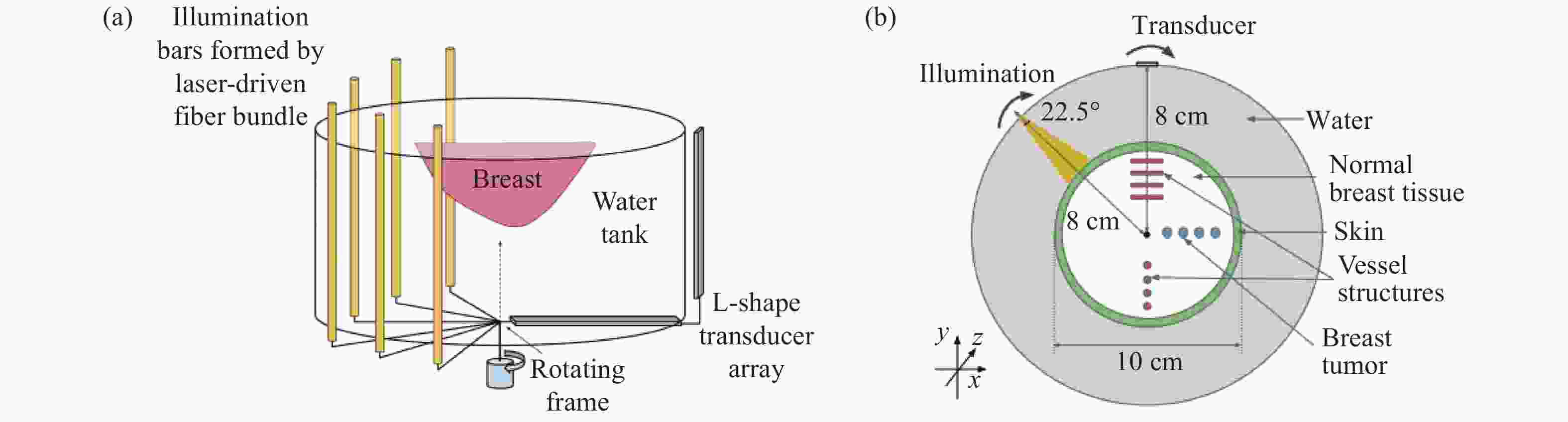
的光吸收系数的估算误差系统分辨率低,校准函数简单,
漫反射图像可能发生畸变,校准
方程不适用于气泡分辨率要求低、目标中气泡含量少
且光吸收系数误差范围大的
静态目标成像表 2 精准估计PAT光通量的主要方法对比
Table 2. Comparison of main methods for accurately estimating light fluence in PAT
方法 优点 不足 适用范围 DOT 成像精度高 假设Gruneisen系数为常数,增加
成像系统及其操作的复杂度具有相同成分或热力学
特性差异不明显的目标光声-声光
信号组合无需有关组织光学特性的先验知识 假设光子的平均自由程大于标记体的线性
维数,间接测量光子功率会引入误差光学特性未知的目标 表面光增强 同步估计光吸收系数、光散射系数和
Gruneisen系数,估计精度高无法确定最小化问题的唯一性 热力学特性差异明显的目标 表 3 PAT照明模式的比较
Table 3. Comparison of two schemes of illumination in PAT
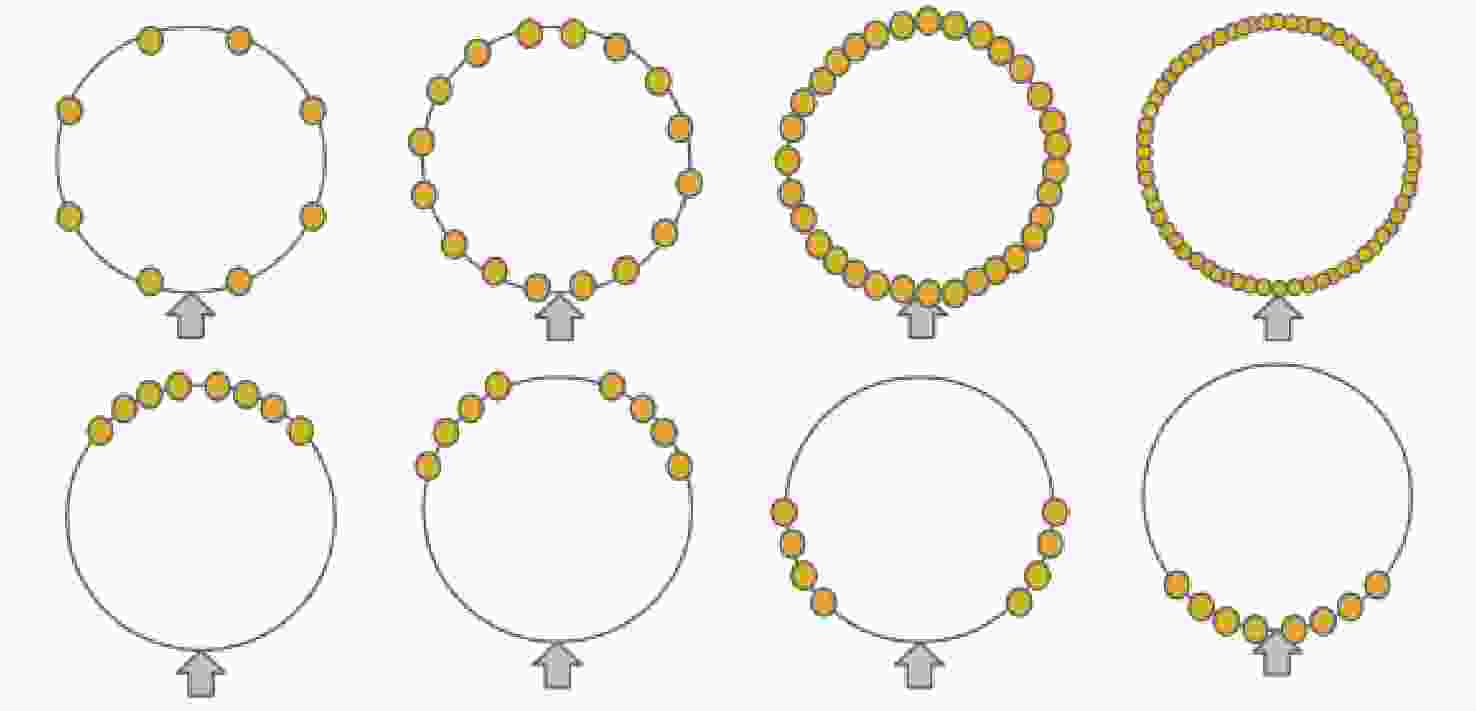
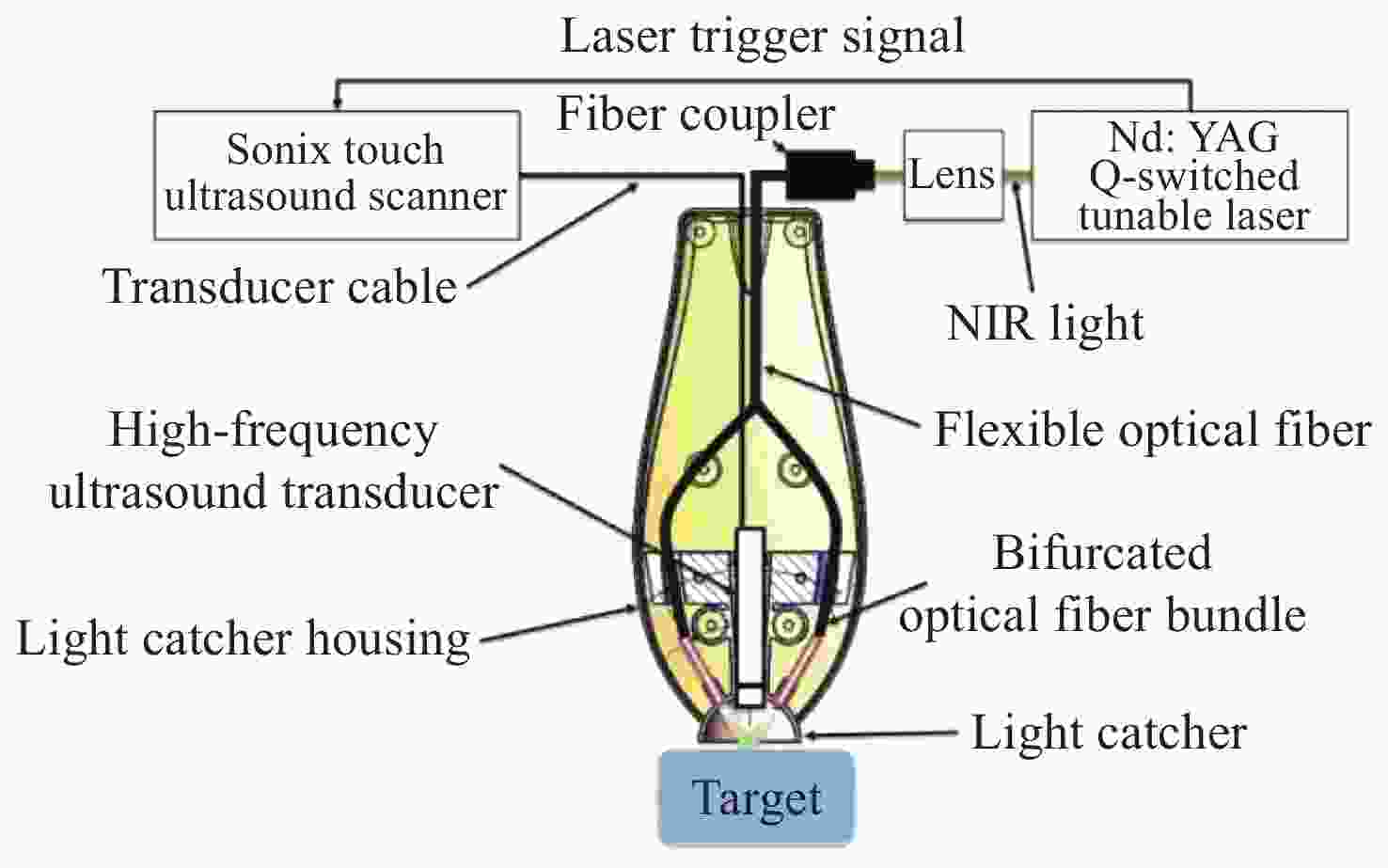
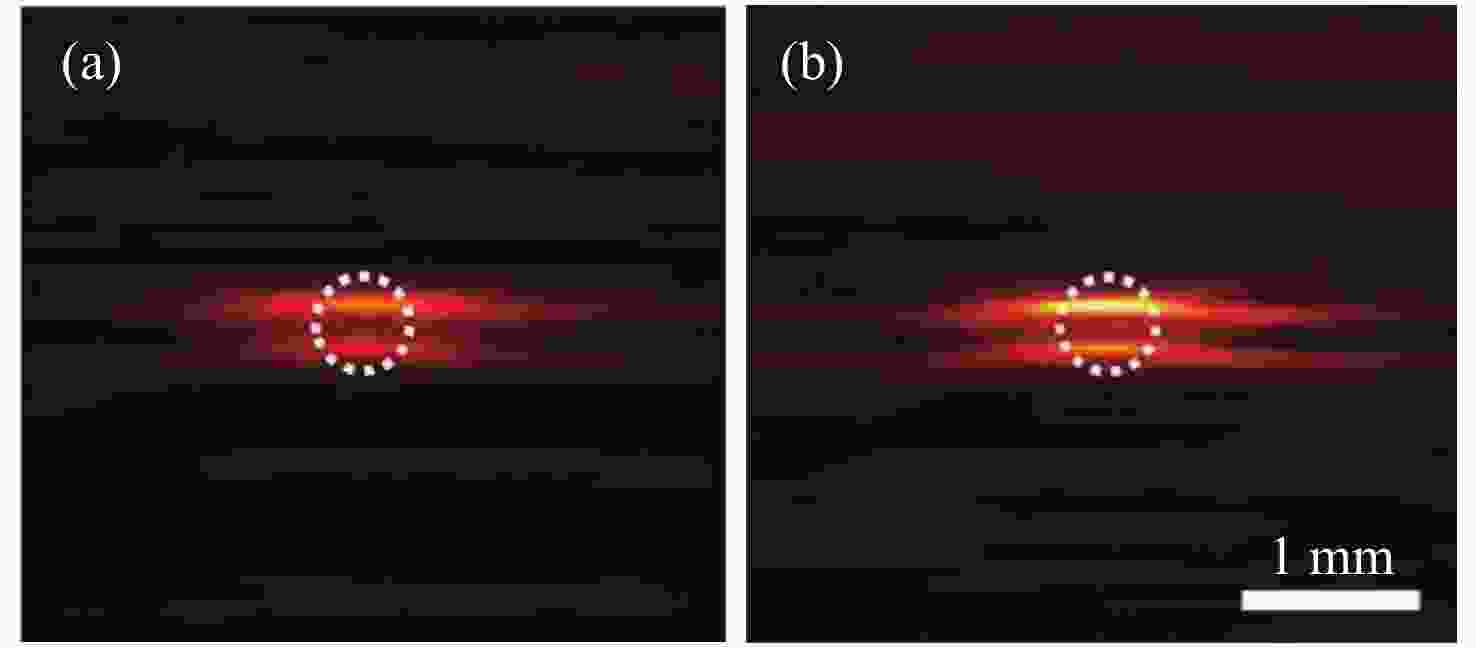
照明模式 优点 不足 适用范围 外部照明 旋转照明 光穿透性强 数据不一致性 体积较小的目标 非平稳照明 改善数据的不一致性,成像深度大 组织内部光束重叠 体积较小的目标 光捕捉器
增强照明解决表面光反射问题,样品表面光照相对均匀,改善深层组织的成像质量 对成像质量的改善有限,很难
用于表面不平坦的目标表面平坦的目标 优化的三维
PAT照明样品表面光照均匀,成像深度大 设备灵活性差,重建精度受限 孤立目标的三维PAT 内部照明 光纤扩散器照明 成像深度高,系统兼容性强 穿透深度受到光纤扩散器与组织
之间光衰减的限制,难以实现
特定方向的照明大型目标,内部器官的成像 -
[1] WANG L V, YAO J J. A practical guide to photoacoustic tomography in the life sciences[J]. Nature Methods, 2016, 13(8): 627-638. doi: 10.1038/nmeth.3925 [2] DELAZEROA A, PAULUS Y M, TEED R, et al. Photoacoustic ocular imaging[J]. Optics Letters, 2010, 35(3): 270-272. doi: 10.1364/OL.35.000270 [3] LI JW, XIAO H, YOON S J, et al. Functional photoacoustic imaging of gastric acid secretion using pH-responsive polyaniline nanoprobes[J]. Small, 2016, 12(34): 4690-4696. doi: 10.1002/smll.201601359 [4] WANG X D, PANG Y J, KU G, et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain[J]. Nature Biotechnology, 2003, 21(7): 803-806. doi: 10.1038/nbt839 [5] ISKANDER-RIZK S, VAN DER STEEN A F W, VAN SOEST G. Photoacoustic imaging for guidance of interventions in cardiovascular medicine[J]. Physics in Medicine &Biology, 2019, 64(16): 16TR01. [6] POUDEL J, LOU Y, ANASTASIO M A. A survey of computational frameworks for solving the acoustic inverse problem in three-dimensional photoacoustic computed tomography[J]. Physics in Medicine &Biology, 2019, 64(14): 14TR01. [7] SPADIN F, JAEGER M, NUSTER R, et al. Quantitative comparison of frequency-domain and delay-and-sum optoacoustic image reconstruction including the effect of coherence factor weighting[J]. Photoacoustics, 2020, 17: 100149. doi: 10.1016/j.pacs.2019.100149 [8] WANG B, WEI N N, PENG K, et al. Modified back-projection method in acoustic resolution based photoacoustic endoscopy for improved lateral resolution[J]. Medical Physics, 2018, 45(10): 4430-4438. doi: 10.1002/mp.13129 [9] ZHENG S, HAN D D, YUAN Y. 2-D image reconstruction of photoacoustic endoscopic imaging based on time-reversal[J]. Computers in Biology and Medicine, 2016, 76: 60-68. doi: 10.1016/j.compbiomed.2016.06.028 [10] WANG K, ANASTASIO M A. A simple Fourier transform-based reconstruction formula for photoacoustic computed tomography with a circular or spherical measurement geometry[J]. Physics in Medicine &Biology, 2012, 57(23): N493-499. [11] COX B T, LAUFER J G, BEARD P C, et al. Quantitative spectroscopic photoacoustic imaging: a review[J]. Journal of Biomedical Optics, 2012, 17(6): 061202. doi: 10.1117/1.JBO.17.6.061202 [12] JAVAHERIAN A, HOLMAN S. Direct quantitative photoacoustic tomography for realistic acoustic media[J]. Inverse Problems, 2019, 35: 084004. doi: 10.1088/1361-6420/ab091e [13] 林剑萍, 廖一鹏. 结合分数阶微分及Retinex的NSCT自适应低照度图像增强[J]. 液晶与显示,2020,35(4):360-373. doi: 10.3788/YJYXS20203504.0360LIN J P, LIAO Y P. NSCT adaptive low illumination image enhancement combining fractional differential and retinex[J]. Chinese Journal of Liquid Crystals and Displays, 2020, 35(4): 360-373. (in Chinese) doi: 10.3788/YJYXS20203504.0360 [14] COX B T, ARRIDGE S R, KÖSTLI K P, et al. Two-dimensional quantitative photoacoustic image reconstruction of absorption distributions in scattering media by use of a simple iterative method[J]. Applied Optics, 2006, 45(8): 1866-1875. doi: 10.1364/AO.45.001866 [15] ROSENTHAL A, RAZANSKY D, NTZIACHRISTOS V. Quantitative optoacoustic signal extraction using sparse signal representation[J]. IEEE Transactions on Medical Imaging, 2009, 28(12): 1997-2006. doi: 10.1109/TMI.2009.2027116 [16] BU SH H, LIU ZH B, SHIINA T, et al. Model-based reconstruction integrated with fluence compensation for photoacoustic tomography[J]. IEEE Transactions on Biomedical Engineering, 2012, 59(5): 1354-1363. doi: 10.1109/TBME.2012.2187649 [17] DORAN A E, HIRATA S. Monte Carlo second- and third-order many-body green’s function methods with frequency-dependent, nondiagonal self-energy[J]. Journal of Chemical Theory and Computation, 2019, 15(11): 6097-6110. doi: 10.1021/acs.jctc.9b00693 [18] 邓衍亚, 李伟伟, 林继, 等. 三维高频声波的矩阵压缩边界节点法模拟[J]. 力学季刊,2019,40(1):32-38.DENG Y Y, LI W W, LIN J, et al. Simulation of three-dimensional high frequency acoustic wave by matrix compression boundary node method[J]. Chinese Quarterly of Mechanics, 2019, 40(1): 32-38. (in Chinese) [19] DEÁN-BEN X L, STIEL A C, JIANG YY, et al. Light fluence normalization in turbid tissues via temporally unmixed multispectral optoacoustic tomography[J]. Optics Letters, 2015, 40(20): 4691-4694. doi: 10.1364/OL.40.004691 [20] HUSSAIN A, PETERSEN W, STALEY J, et al. Quantitative blood oxygen saturation imaging using combined photoacoustics and acousto-optics[J]. Optics Letters, 2016, 41(8): 1720-1723. doi: 10.1364/OL.41.001720 [21] MIZEVA I, DREMIN V, POTAPOVA E, et al. Wavelet analysis of the temporal dynamics of the laser speckle contrast in human skin[J]. IEEE Transactions on Bio-medical Engineering, 2020, 67(7): 1882-1889. [22] 钱伟, 蒋明. 数字图像相关方法中数字散斑场的制作与应用研究[J]. 液晶与显示,2020,35(8):861-869. doi: 10.37188/YJYXS20203508.0861QIAN W, JIANG M. Design and application of digital speckle patterns in digital image correlation method[J]. Chinese Journal of Liquid Crystals and Displays, 2020, 35(8): 861-869. (in Chinese) doi: 10.37188/YJYXS20203508.0861 [23] ZHAO L Y, YANG M, JIANG Y X, et al. Optical fluence compensation for handheld photoacoustic probe: an in vivo human study case[J]. Journal of Innovative Optical Health Sciences, 2017, 10(4): 1740002. doi: 10.1142/S1793545817400028 [24] JIN H R, ZHANG R C, LIU Y, et al. A single sensor dual-modality photoacoustic fusion imaging for compensation of light fluence variation[J]. IEEE Transactions on Biomedical Engineering, 2019, 66(6): 1810-1813. doi: 10.1109/TBME.2019.2904502 [25] JIN H R, ZHANG R C, LIU Y, et al. Passive ultrasound aided acoustic resolution photoacoustic microscopy imaging for layered heterogeneous media[J]. Applied Physics Letters, 2018, 113(24): 241901. doi: 10.1063/1.5064417 [26] MOOTHANCHERY M, BI R ZH, KIM J Y, et al. Optical resolution photoacoustic microscopy based on multimode fibers[J]. Biomedical Optics Express, 2018, 9(3): 1190-1197. doi: 10.1364/BOE.9.001190 [27] MOOTHANCHERY M, DEV K, BALASUNDARAM G, et al. Acoustic resolution photoacoustic microscopy based on microelectromechanical systems scanner[J]. Journal of Biophotonics, 2020, 13(2): e201960127. [28] BAUER A Q, NOTHDURFT RE, CULVER JF, et al. Quantitative photoacoustic imaging: correcting for heterogeneous light fluence distributions using diffuse optical tomography[J]. Journal of Biomedical Optics, , 2011, 16(9): 096016. doi: 10.1117/1.3626212 [29] MAHMOODKALAYEH S, ZAREI M, ANSARI M A, et al. Improving vascular imaging with co-planar mutually guided photoacoustic and diffuse optical tomography: a simulation study[J]. Biomedical Optics Express, 2020, 11(8): 4333-4347. doi: 10.1364/BOE.385017 [30] DAOUDI K, MOLENAAR R, VANLEEUWEN T G, et al. Absolute measurement of absorption coefficient by combining photoacoustics and acousto-optics[C]. Proceedings of SPIE International Conference on Photons Plus Ultrasound: Imaging and Sensing 2011, 2011, 7899: 78990V. [31] DAOUDI K, HUSSAIN A, HONDEBRINK E, et al. Correcting photoacoustic signals for fluence variations using acousto-optic modulation[J]. Optics Express, 2012, 20(13): 14117-14129. doi: 10.1364/OE.20.014117 [32] HUSSAIN A, DAOUDI K, HONDEBRINK E, et al.. Quantitative photoacoustic imaging by acousto-optically measured light fluence[C]. In Biomedical Optics and 3-D Imaging, OSA Technical Digest (Optical Society of America, 2012), OSA, 2012. [33] STEENBERGEN W, MOLENAAR R, DAOUDI K. Combined application of photoacoustic and acousto-optic imaging for model-free quantitative optical absorption mapping[J]. The Journal of the Acoustical Society of America, 2011, 129(4): 2641. [34] STEENBERGEN W. Towards quantitative imaging of absorption coefficients in turbid media by combining photoacoustic and acousto-optic imaging[C]. Biomedical Optics and 3-D Imaging, OSA Technical Digest (CD) (Optical Society of America, 2010), 2010. [35] NYKÄNEN O, PULKKINEN A, TARVAINEN T. Quantitative photoacoustic tomography augmented with surface light measurements[J]. Biomedical Optics Express, 2017, 8(10): 4380-4395. doi: 10.1364/BOE.8.004380 [36] LOU Y, NADVORETSKIY V, WANG K, et al.. Effect of rotating partial illumination on image reconstruction for optoacoustic breast tomography[J]. Proceedings of SPIE, 2015, 9323: 93233L. [37] LOU Y, WANG K, ORAEVSKY A A, et al. Impact of nonstationary optical illumination on image reconstruction in optoacoustic tomography[J]. Journal of the Optical Society of America A, 2016, 33(12): 2333-2347. [38] PARK S, ORAEVSKY A A, SU R, et al.. Compensation for non-uniform illumination and optical fluence attenuation in three-dimensional optoacoustic tomography of the breast[J]. Proceedings of SPIE, 2019, 10878: 108784X. [39] YU J, JUNG Y, KANG J, et al. Enhancement of photoacoustic signal using a novel light illumination improvement device: in vivo feasibility animal study[C]. Proceedings of 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3-6 Sept. 2014: 349-352. [40] YU J, SCHUMAN J S, LEE J K, et al. A light illumination enhancement device for photoacoustic imaging: in vivo animal study[J]. IEEE Transactions on Ultrasonics,Ferroelectrics,and Frequency Control, 2017, 64(8): 1205-1211. doi: 10.1109/TUFFC.2017.2713599 [41] LARNEY B M, REBLING J, CHEN ZH Y, et al. Uniform light delivery in volumetric optoacoustic tomography[J]. Journal of Biophotonics, 2019, 12(6): e201800387. [42] LI M C, LAN B X, LIU W, et al. Internal-illumination photoacoustic computed tomography[J]. Journal of Biomedical Optics, 2018, 23(3): 1-4. [43] JOHNSTONBAUGH K, AGRAWAL S, ABHISHEK D, et al.. Novel deep learning architecture for optical fluence dependent photoacoustic target localization[J]. Proceedings of SPIE, 2019, 10878: 108781L. [44] HARIRI A, ALIPOUR K, MANTRI Y, et al. Deep learning improves contrast in low-fluence photoacoustic imaging[J]. Biomedical Optics Express, 2020, 11(6): 3360-3373. doi: 10.1364/BOE.395683 [45] CHEN T T, LU T, SONG SH Z, et al.. A deep learning method based on U-Net for quantitative photoacoustic imaging[J]. Proceedings of SPIE, 2020, 11240: 112403V. [46] GORE J C. Artificial intelligence in medical imaging[J]. Magnetic Resonance Imaging, 2020, 68: A1-A4. doi: 10.1016/j.mri.2019.12.006 [47] 王慧, 冯金顺, 程正兴. 基于局部路径特征信息神经网络的图像去噪[J]. 液晶与显示,2020,35(1):70-79. doi: 10.3788/YJYXS20203501.0070WANG H, FENG J SH, CHENG ZH X. Image denoising based on local path feature in formation neural network[J]. Chinese Journal of Liquid Crystals and Displays, 2020, 35(1): 70-79. (in Chinese) doi: 10.3788/YJYXS20203501.0070 -





 下载:
下载: